
ijУ̽��ѧϰС��ͬѧ�ú�����������(��ҪΪ������ɳ��CaCl2��MgCl2��Na2SO4��)�Ĵ�����ȡ����ѧ��������NaCl��ʵ��ǰ������������·���(��ͼ)�� 
(1)��д�������ڢܡ��ݲ������Լ����Ƽ��ڢ��������ƣ��� ���� ���� ��
(2) ���������C�Ļ�ѧ�ɷ���(�����ֺͻ�ѧʽ��ʾ)�� ��
(3)д���ڢݲ������п��ܷ�����Ӧ�����ӷ���ʽ��
��
(4)��������������ڢݲ�ʵ���Ƿ�ﵽ��Ŀ�ģ�
��
(5)����Ϊ���������Щ���������Ӱ��ʵ������ ��
(6)��ͬѧ��Ϊ����ʵ����Ʋ�����Լ����������һ�����룺
��
�Ţ�̼������Һ ������ �������������ᾧҲ�ɣ�����1�֣�
�� ��ɳ��BaSO4��BaCO3��CaCO3��Mg(OH)2��2�֣�
�ǵڢݲ���H+ + OH- = H2O ��1�֣� CO32- + 2H+ = H2O + CO2����1�֣�
�� �ò�����պȡ��Һ����pH��ֽ�в�������죬˵����Һ�����ԣ���OH-��CO32-���ڣ���������˵����Һ�Գʼ��ԣ���OH-��CO32-���ڣ�������μ������������ԡ���2�֣�
�ɢں͢ۻ�ۺ͢ܣ�2�֣�
�� ��Ba (OH)2����BaCl2��NaOH��ʹ�ڢ۲��ϲ�Ϊһ����1�֣�
���������������1����������ͼ�Ѹ������Լ������ã�BaCl2��Һ��ȥNa2SO4��NaOH��Һ��ȥMgCl2�����Ԣܵ�����Ϊ��ȥCaCl2����ѡ�Լ�ΪNa2CO3��Һ���ݵ������dz�ȥ������NaOH��Na2CO3��Ӧѡ�����ᣬ�������Ǵ�NaCl��Һ�еõ���ѧ��NaCl��Ϊ����������
��2��BaCl2��NaOH��Na2CO3��Ӧ��õ��ij������У���ɳ��BaSO4��BaCO3��CaCO3��Mg(OH)2��
��3���ڢݲ�����HCl����NaOH��Na2CO3��Ӧ���������ӷ���ʽΪ��H+ + OH- = H2O�� CO32- + 2H+ = H2O + CO2����
��4��HCl����ʱ�ڢݲ��ﵽ��Ŀ�ģ�����pH��ֽ���飬����ֽ�仯��֤��HCl������OH?��CO32?�����ڣ���������˵������OH?��CO32?��������μ������������ԡ�
��5��NaOH��Ӱ���������ʵij�ȥ������NaOH�ļ���˳�������ǰ��Ҳ���������������ں͢ۻ�ۺܿ͢ɵ�����
��6����Ba (OH)2����BaCl2��NaOH����ͬʱ��ȥNa2SO4��MgCl2��ʹ�ڢ۲��ϲ�Ϊһ����
���㣺���⿼������ͼ�ķ������Լ���ѡ�������������ӷ���ʽ����д��ʵ�鷽������ƺͷ�����


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС�齫һ��Ũ��Na2CO3��Һ����CuSO4��Һ�еõ���ɫ������
��ͬѧ��Ϊ���߷�Ӧֻ����CuCO3һ�ֳ�����
��ͬѧ��Ϊ��������ٽ�ˮ�ⷴӦ������һ��Cu(OH)2������
��ͬѧ��Ϊ����CuCO3��Cu(OH)2���ֳ�����
����������֪��CuCO3��Cu(OH)2�������ᾧˮ��
��.������ͬѧ������Na2CO3��Һ��CuSO4��Һ��Ӧ�����ӷ�Ӧ����ʽΪ�� _ ��
��̽��������ɷ�ǰ���뽫��������Һ�з��벢�������������Ϊ �ٹ��ˢ�ϴ�Ӣ۸��
��������ͼ��ʾװ�ã�ѡ���Ҫ���Լ�������̽��������ijɷ֡�
��1����װ������˳��Ϊ 
 ��
��
��2��װ��C��װ�е��Լ������� ��
��3�������װ������˳�IJ�������ȷ�ģ����ͨ��________________________������˵���IJ�������ȷ�ġ�
����CuCO3��Cu(OH)2���߶��У���ͬѧͨ��װ�â���ж����������ⶨ����ɡ�
��1��ʵ���йز���Ϊ��������ƿ�з�����������Ʒ���ڴӷ�Һ©������ƿ�м��������ϡ����ۼ���װ�õ������ԣ��ܲⶨ�ռ���ˮ�������
��ȷ�IJ���˳��Ϊ____________________��
��2�����ƿ��ˮ�����渲����һ��ֲ���ͣ���ƿ�е�Һ��û��װ�����Ϸ����������ռ䣩��ʵ����CO2�������___________����ƫ��ƫС�䣩��
��3����ʵ���в����Ʒ������Ϊwg����������ΪaL������£�������Ʒ��CuCO3����������Ϊ___________��CuCO3��ʽ��Ϊ124����
��4����ͬѧ��Ϊ����ͬѧ��ʵ�鷽��������������������������������ϴ������ͼ�еĢ�IIIװ�ý���ʵ�顣ֻ����м��ֱ�Ҫ�����ݲⶨ���ɱȽ�ȷ��ȷ����Ʒ��CuCO3�������������Ϻ�����װ��_______������ţ������Ƕ�ͬѧ��װ����Ȼ��ȱ�ݣ���������ƫ��ƫ��__________��������___________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�ۺ��������ֳƾ�������ѧʽΪ[Fe2(OH)n(SO4)3��0.5n]m�� �㷺������ˮ������ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ��������̷�(FeSO4��7H2O)���������£�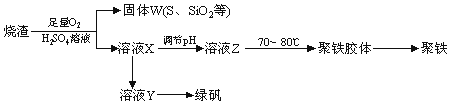
��1����֤����W���պ���������庬��SO2�ķ����ǣ�________________________��
��2��ʵ�����Ʊ����ռ������SO2�������������¡�װ��A����SO2���������������Ӹ������ӿڣ�˳��Ϊa��____��____��____��____��f��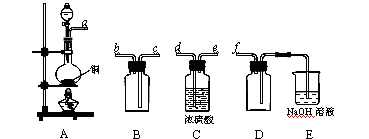
װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ_________________________________________��
��3���Ʊ��̷�ʱ������ҺX�м������__________����ַ�Ӧ�����˲����õ���ҺY���پ�Ũ�����ᾧ�Ȳ���õ��̷�����������IJ���������_____________________��
��4�����ⶨ��ҺY��Fe2+��Ũ�ȣ���Ҫ������ƿ����KMnO4����Һ����KMnO4����Һ�ζ�ʱӦѡ��________�ζ���(���ʽ����ʽ��)��
��5����ҺZ��pHӰ�������������������������ҺZ��pHƫС�������¾�����������������______(�ƫ����ƫС������Ӱ�족)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ������ȡ���ᶡ����ʵ��װ�������¼ס�������װ�ÿɹ�ѡ�á�

���ף� ���ң�
�Ʊ����ᶡ�����漰���й����ʵ��������ʼ��±�
| | ���� | 1������ | ���ᶡ�� |
| �۵�(��) | 16��6 | ��89��5 | ��73��5 |
| �е�(��) | 117��9 | 117 | 126��3 |
| �ܶ�(g/cm3) | 1��05 | 0��81 | 0��88 |
| ˮ���� | ���� | ���� (9g/100gˮ) | �� |





�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��.��1��ij��ѧ��ȤС����������װ����ѡȡ��Ҫ��װ����ȡ��NH4��2SO4��Һ�����ӵ�˳���ýӿ������ĸ��ʾ���ǣ�a ��
��2����װ��C������Һ����뿪�IJ��������� ��װ��D�������� ��
��.�������ƿ������ڸ��Ƶر�ˮ�ʡ��������ؽ������ӷ�ˮ�������ೱ��Ҳ������Ӧ�������ȡ���ҵ�������������Ƶ���Ҫ�������£�
��֪CaO2��8H2O�ʰ�ɫ������ˮ��I2+2S2O32��= 2I��+S4O62��
��1��������������ȡCaO2��8H2O�Ļ�ѧ����ʽ�� ��
��2�����顰ˮϴ���Ƿ�ϸ�ķ����� ��
��3���ⶨ��Ʒ��CaO2�ĺ�����ʵ�鲽���ǣ�
��һ����ȷ��ȡa g��Ʒ����ƿ�У�������������ˮ������b g KI���壬�ٵ�������2 mol/L��H2SO4��Һ����ַ�Ӧ��
�ڶ�������������ƿ�м��뼸�ε�����Һ��
����������μ���Ũ��Ϊc mol��L��1��Na2S2O3��Һ����Ӧ��ȫ������Na2S2O3��ҺV mL��
���жϴ˵ζ�ʵ��ﵽ�յ�ķ����ǣ� ��
��CaO2����������Ϊ (����ĸ��ʾ)��
��ijͬѧ��һ���͵ڶ����IJ������ܹ淶������������̫����������õ�CaO2�������������� �������Ӱ�족����ƫ�͡���ƫ�ߡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
I����ͼ��ʾ�ӹ��������з���Q��2�ַ�������ش��й����⡣
��1��ѡ�÷���(i)ʱ��QӦ�þ��е�������_____________��������Ӧ�þ�
�������__________________________________��
��2��ѡ�÷���(ii)��ij������ĩ������Au��Ag��Cu���з���Au��������Լ�Ϊ____________��
��3��Ϊ�ᴿijFe2O3��Ʒ����Ҫ������SiO2.Al2O3�������շ���(i)��(ii)�������һ���Կ�ͼ��ʽ��ʾ��ʵ�鷽����ע�����ʺͲ�������
______________________________________________________________________________��
��ij�ֺ������������ƵĹ���������Ʒ����֪��Ʒ����Ϊ1.560g����ƿ��ˮ������Ϊ
190.720g)����������ͼ��ʾװ�òⶨ�������Na2O2������������ÿ����ͬʱ����õ�
����ƽ���������±���
��4��д��Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ��________________________________________��
��5������Na2O2��������ʱ�������������_________________________________________.
��������6�ζ�����ԭ����_____________________________________________________��
��6���ⶨ������Ʒ(1.560g)��Na2O2������������һ�ַ�����������������£�
�����ڵ�������____________���÷�����ֱ�Ӳⶨ����������_____________ ���ⶨ������
��Ҫ�������е�����ƽ�������ƾ��ƣ�����Ҫ___________��__________���̶�����
���������⣩,��ת����Һʱ������Һת�Ʋ���ȫ����Na2O2���������IJⶨ���_______����
��ƫ����ƫС�����䡱����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��������泥�NH2COONH4����һ�ְ�ɫ���壬�ֽ⡢��ˮ�⣬���������ϡ�������ϴ�Ӽ��ȡ�ij��ѧ��ȤС��ģ���Ʊ���������泥���Ӧ�Ļ�ѧ����ʽ���£�
2NH3(g)+CO2(g) NH2COONH4(s) + Q (Q > 0 )
NH2COONH4(s) + Q (Q > 0 )
��1��������ͼװ����ȡ����������ѡ����Լ��� ��
�Ʊ���������淋�װ������ͼ��ʾ���Ѱ����Ͷ�����̼ͨ�����Ȼ�̼�У����Ͻ����ϣ����ɵİ��������С�������������Ȼ�̼�С� ��������϶�ʱ��ֹͣ�Ʊ���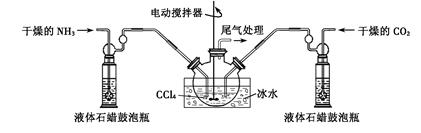
ע�����Ȼ�̼��Һ��ʯ����Ϊ���Խ��ʡ�
��2���������ñ�ˮ��ȴ��ԭ����___________ __ _��
��3��Һ��ʯ������ƿ��������_______��
��4���ӷ�Ӧ��Ļ�����з������Ʒ��ʵ�鷽����_______����д�������ƣ���Ϊ�˵õ������Ʒ��Ӧ��ȡ�ķ�����________����дѡ����ţ���
a. ��ѹ���Ⱥ�� b. ��ѹ���Ⱥ�� c. ���40 �����º��
��5��β������װ����ͼ��ʾ��˫ͨ�����ܵ����ã�________ ��Ũ��������ã� ��______________ _��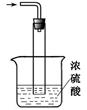
��6��ȡ�ֱ��ʶ�����̼����淋İ����������Ʒ0.7820 g��������ʯ��ˮ��ִ�����ʹ̼Ԫ����ȫת��Ϊ̼��ƣ����ˡ�ϴ�ӡ�����������Ϊ1.000 g������Ʒ�а�������淋����ʵ�������Ϊ
___________������ȷ��2λС����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
̼�����׳ƴ������;�ܹ㡣ʵ�����У���̼����狀ͱ���ʳ��ˮ���Ƶô���������ڲ�ͬ�¶��µ��ܽ�ȼ�����
  �¶ȡ� �¶ȡ��ܽ�� ���� g/100gˮ | 10 | 20 | 30 | 40 | 50 | 60 | 70 |
| NaCl | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 37.8 |
| NH4HCO3 | 15.8 | 21.0 | 27.0 | | | | |
| NaHCO3 | 8.2 | 9.6 | 11.1 | 12.7 | 14.4 | 16.4 | |
| NH4Cl | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 55.2 | 60.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
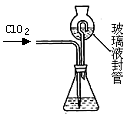
�������ȣ�ClO2����Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч�����Ĺ�������������һ�ֻ���ɫ�����壬������ˮ��ʵ���ҿ���NH4Cl�����ᡢNaClO2���������ƣ�Ϊԭ�����Ʊ�ClO2�����������£�
��1��д�����ʱ������Ӧ�Ļ�ѧ����ʽ�� ��
��2����ȥClO2�е�NH3��ѡ�õ��Լ��� ��������ĸ��
| A������ʳ��ˮ | B����ʯ�� | C��Ũ���� | D��ˮ |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| �� | | ��ҺX�к���Na+ |
| �� | | ��ҺX�к���Cl�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com