
����Ŀ�������£���һԪ��HA����Һ��KOH��Һ��������(��������仯)��ʵ���������±��������жϲ���ȷ����

A. ʵ��ٷ�Ӧ�����Һ�У�c(K��)��c(A��)��c(OH��)��c(H��)
B. 0.1 mol��L��1HA����Һ����ˮ�������c(H��)��1��10��13 mol��L��1
C. ʵ��ڷ�Ӧ�����Һ�У�c(A��)��c(HA)��0.1 mol��L��1
D. ʵ��ڷ�Ӧ�����Һ�У�c(K��)��c(A��)��c(OH��)��c(H��)
���𰸡�B
��������
A��0.1 mol��L��1��HA����Һ��0.1 mol��L��1��KOH��Һ�������ϣ�ǡ����ȫ��Ӧ����KA����Ӧ����ҺpH=9��˵��KA��ǿ����������A��ˮ����B��HA��������0.1 mol��L��1HA����Һ��������������Ũ��С��0.1 mol��L��1��C����x=0.2������Һ�ʼ���������x>0.2�����ݵ���غ㣬c(K��)+ c(H��)= c(A��)+c(OH��)��pH=7��˵��c(OH��)��c(H��)������c(K��)��c(A��)��
0.1 mol��L��1��HA����Һ��0.1 mol��L��1��KOH��Һ�������ϣ�ǡ����ȫ��Ӧ����KA����Ӧ����ҺpH=9��˵��KA��ǿ����������A��ˮ��������ʵ��ٷ�Ӧ�����Һ�У�c(K��)��c(A��)��c(OH��)��c(H��)����A��ȷ��HA��������0.1 mol��L��1HA����Һ��������������Ũ��С��0.1 mol��L��1��������ˮ�������c(H��)>1��10��13 mol��L��1����B����C����x=0.2������Һ�ʼ���������x>0.2�����������غ���c(A��)��c(HA)��0.1 mol��L��1����C��ȷ�����ݵ���غ㣬c(K��)+ c(H��)= c(A��)+c(OH��)��pH=7��˵��c(OH��)��c(H��)������c(K��)��c(A��)��c(K��)��c(A��)��c(OH��)��c(H��)����D��ȷ��


 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)______mol H2O�к��е���ԭ������1.5 mol CO2�к��е���ԭ������ȡ�
(2)�������ʵ�����NH3��CH4��ϣ����������NH3��CH4��������Ϊ________��
(3)ҪʹNH3��CH4����ͬ��Ŀ��Hԭ�ӣ���NH3��CH4�����ʵ���֮��Ϊ__________��
(4)�������ǵ��������ı����������������и�ǿ��������.ʵ���ҿɽ�����ͨ����ѹ�ŵ������ȡ������![]()
![]()
![]()
�� ����������Ӧ����30 %������ת��Ϊ���������û������ƽ��Ħ������Ϊ________![]() (����һλС��)
(����һλС��)
�ڽ�8L����ͨ���ŵ�ָܺ���ԭ״�����õ�����6.5L�����г���Ϊ__________L
��ʵ���ҽ������ͳ����Ļ������0.896L (��״��)ͨ��ʢ��20.0gͭ�۵ķ�Ӧ���У���ּ��Ⱥ���̬ȫ���μӷ�Ӧ������ĩ��������Ϊ21.6g����ԭ������г������������Ϊ__________���û�����������������ܶ�Ϊ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��2 mol O3��3 mol O2������֮��Ϊ________��������֮��Ϊ________��ͬ��ͬѹ�µ��ܶ�֮��Ϊ________������ԭ����֮��Ϊ________�����֮��Ϊ________��
��2���ڱ�״���£���CO��CO2��ɵĻ������6.72 L������Ϊ12 g���˻������CO��CO2���ʵ���֮����______����������ƽ����Է���������_______��������������ܶ���________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������һ����Ҫ�����ȼ�ϣ�����ͨ��CH3OH���Ӽ���ˮ�Ƶã�2CH3OH(g) ![]() CH3OCH3(g) �� H2O(g) ��H����23.5kJ��mol��1����t1���������ܱ������н�������ƽ�⣬��ϵ�и����Ũ����ʱ��仯��ͼ��ʾ��
CH3OCH3(g) �� H2O(g) ��H����23.5kJ��mol��1����t1���������ܱ������н�������ƽ�⣬��ϵ�и����Ũ����ʱ��仯��ͼ��ʾ��

(1)�������·�Ӧƽ�ⳣ������ʽK��______________����t1 ��ʱ����Ӧ��ƽ�ⳣ��Ϊ____________���ﵽƽ��ʱn(CH3OCH3)��n(CH3OH)��n(H2O)=_____________________��
(2)��ͬ�����£����ı���ʼŨ�ȣ�ijʱ�̸����Ũ������Ϊ��c(CH3OH)��0.4 mol��L��1��c(H2O)��0.6 mol��L��1��c(CH3OCH3)��2.4mol��L��1����ʱ�����淴Ӧ���ʵĴ�С��v��________v��(����>������<����������)����Ӧ��__________��Ӧ�������(����������������)����
������֪���淴Ӧ��M(g)��N(g) ![]() P(g)��Q(g) ��H��0����ش��������⣺
P(g)��Q(g) ��H��0����ش��������⣺
(1)��ij�¶��£���Ӧ�����ʼŨ�ȷֱ�Ϊc(M)��1 mol��L��1��c(N)��2.4 mol��L��1���ﵽƽ���M��ת����Ϊ60%����ʱN��ת����Ϊ____________��
(2)����Ӧ�¶����ߣ�M��ת����__________(����������������С������������)��
(3)����Ӧ�¶Ȳ��䣬��Ӧ�����ʼŨ�ȷֱ�Ϊc(M)��4 mol��L��1��c(N)��a mol��L��1���ﵽƽ���c(P)��2 mol��L��1��a��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
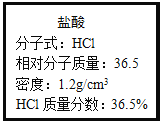
��1����Ũ��������ʵ���Ũ��Ϊ___��
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����___��
A.��Һ��HCl�����ʵ��� B.��Һ��Ũ��
C.��Һ��Cl-����Ŀ D.��Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����450mL���ʵ���Ũ��Ϊ0.3mol/Lϡ���ᡣ
�ٸ�ѧ����Ҫ��ȡ____mL����Ũ����������ơ�
������ʱ������ȷ�IJ���˳����(����ĸ��ʾ��ÿ����ĸֻ����һ��)___��
A.��30mLˮϴ���ձ�2~3�Σ�ϴ��Һ��ע������ƿ����
B.����Ͳȷ��ȡ����Ũ���������������ر���ע��ʢ������ˮ(Լ30mL)���ձ��У��ò���������������ʹ���Ͼ���
C.������ȴ�������ز�����ע��500mL������ƿ��
D.������ƿ�ǽ����ߵ�ҡ��
E.���ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�������
F.����������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1~2cm��
�������ƹ����У�����ʵ������������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿(����ƫ��������ƫ����������Ӱ����)��
I������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ��____
II����Һע������ƿǰû�лָ������¾ͽ��ж���___
��4�����ڱ�״���£���VLHCl��������1Lˮ�У�������Һ�ܶ�Ϊdg/mL�������Һ�����ʵ���Ũ��Ϊ___mol��L��1(����ĸ)
A.![]() B.
B.![]() C.
C.![]() D.
D.![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����25��ʱ�������Ϊ20mLŨ�Ⱦ�Ϊ0.1mol��L��1��������HX��HY��Һ�зքe�μ�0.1mol��L��1��NaOH��Һ����NaOH��Һ����뷴Ӧ����Һ��pH�Ĺ�ϵ��ͼ��ʾ������������ȷ����

A. HX��HY������
B. Ka(HY)��������ԼΪ10��6
C. b��ʱ��2c(Na+)=c(Y��)+c(HY)
D. V(NaOH)=20mLʱ����Ӧ���������Һ��c(X��)=c(Y��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������÷�Һ©�����з����һ��������
A. ������Ҵ�B. ˮ���Ȼ���C. ˮ�ͻ�����D. ���Ȼ�̼�͵�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
��1��д���������������ƣ�
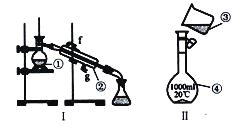
��__________________����__________________����__________________��
��2�������������У�ʹ��ʱ�������Ƿ�©ˮ����_______________������š�
��3����������250 mL0.2mol/LNaCl��Һ��װ��II��ijͬѧת����Һ��ʾ��ͼ��ͼ������������_____________��___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������У�������Cl2ֱ�ӷ�Ӧ�Ƶõ���
A.CuCl2B.FeCl2C.Ca(ClO)2D.NaCl
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com