
| ���� | ���� |
| ����ʢ��4.0gNa2O2���ձ��м���50mL����ˮ | ���ҷ�Ӧ��������������ʹ������ľ����ȼ������ȫ���ܽ�õ�����ɫ��Һa |
| ������Һa�е������η�̪ | ��Һ��죬10���Ӻ���Һ��ɫ���Ա�dz���Ժ���Һ��Ϊ��ɫ |
| ������Һ�м�������MnO2��ĩ | ���д������ݲ���������������Ҳ��ʹ������ľ����ȼ |
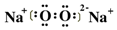 �������ԣ�ʵ��֤ʵ����Һa��H2O2�Ĵ��ڣ�Ӧ��ͬλ��ʾ��ԭ�����Ա�
�������ԣ�ʵ��֤ʵ����Һa��H2O2�Ĵ��ڣ�Ӧ��ͬλ��ʾ��ԭ�����Ա����� ��1��Na2O2Ϊ���ӻ�����ݴ���д����ʽ������ʵ��֤ʵ����Һa��H2O2�Ĵ��ڣ���Na218O2��H2O��Ӧ�������������ƺ�H2O2��H2O2���ٷֽ�����ˮ��������
��2������H2O2����ǿ������Ư�ף�
��3������KMnO4��Һ������HCl��������ϡH2SO4�ữ��KMnO4��Һ����ǿ�����ԣ�Ӧװ����ʽ�ζ����У��յ�ȷ���ķ����ǵ������һ��ʱ��Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ������KMnO4��H2O2��Ӧ�����ʵ���֮�����H2O2�����ʵ��������������Һa��c��H2O2����
��4������Һa�еμ�FeSO4��Һ��Na2O2����FeSO4��Һ������Fe��OH��3����������
��5����FeSO4��Һ�м���һ����Na202���壬�������ʵ���Ϊ2��1������Ӧ����Ӧ�����������ɣ�����Na202���������������FeSO4��Һ��������4Fe��OH��3�����ݴ���д��
��� �⣺��1��Na2O2Ϊ���ӻ���������ʽΪ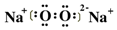 ����ʵ��֤ʵ����Һa��H2O2�Ĵ��ڣ���Na218O2��H2O��Ӧ�������������ƺ�H2O2��H2O2���ٷֽ�����ˮ������������Na218O2��H2O��Ӧ���ܻ�ѧ����ʽΪ2Na218O2+2H2O�T2Na18OH+2NaOH+18O2�����ʴ�Ϊ��
����ʵ��֤ʵ����Һa��H2O2�Ĵ��ڣ���Na218O2��H2O��Ӧ�������������ƺ�H2O2��H2O2���ٷֽ�����ˮ������������Na218O2��H2O��Ӧ���ܻ�ѧ����ʽΪ2Na218O2+2H2O�T2Na18OH+2NaOH+18O2�����ʴ�Ϊ��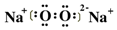 ��2Na218O2+2H2O�T2Na18OH+2NaOH+18O2����
��2Na218O2+2H2O�T2Na18OH+2NaOH+18O2����
��2����ΪH2O2����ǿ������Ư�ף����Բ������к�ɫ��ȥ�Ŀ���ԭ������Һa�й���H2O2���̪������Ӧ���ʴ�Ϊ����Һa�й���H2O2���̪������Ӧ��
��3������KMnO4��Һ������HCl��������ϡH2SO4�ữ��KMnO4��Һ����ǿ�����ԣ�Ӧװ����ʽ�ζ����У��յ�ȷ���ķ����ǵ������һ��ʱ��Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ������KMnO4��H2O2��Ӧ�����ӷ���ʽ��֪��ϵʽΪ��
2MnO4-��5H2O2
2mol 5mol
0.002mol•L-1��0.01L n��H2O2��
��n��H2O2��=5��10-5mol
����c��H2O2��=$\frac{5��10{\;}^{-5}mol}{2��1{0}^{-2}L}$=0.0025mol/L���ʴ�Ϊ��H2SO4�� � �������һ��ʱ��Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ�� 0.0025��
��4������Һa�еμ�FeSO4��Һ��Na2O2����FeSO4��Һ������Fe��OH��3�������������ӷ���ʽΪ��4Na2O2+4Fe2++6H2O=O2��+4Fe��OH��3��+8Na+���ʴ�Ϊ��4Na2O2+4Fe2++6H2O=O2��+4Fe��OH��3��+8Na+��
��5����FeSO4��Һ�м���һ����Na202���壬�������ʵ���Ϊ2��1������Ӧ����Ӧ�����������ɣ�����Na202���������������FeSO4��Һ��������4Fe��OH��3�������ӷ���ʽΪ��3Na2O2+6 Fe2++6H2O=6Na++4Fe��OH��3��+2Fe3+���ʴ�Ϊ��3Na2O2+6 Fe2++6H2O=6Na++4Fe��OH��3��+2Fe3+��
���� ���������ʵ�����Ϊ���������������ӷ���ʽ��д����ѧ���㡢ʵ���������ѧ���ۺ�����Ԫ�ػ�����֪ʶ������Ҫ��ϸߣ��ѶȽϴ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ ���� | B�� | ���� ���� | ||
| C�� | ��ȡ ��Һ | D�� | ��ȡ ��Һ ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��XΪO����Y��X�γɵij���������һ�������ӻ����� | |
| B�� | ��X��Na����Z���������һ����ż�� | |
| C�� | ��YΪO����ǽ����ԣ�X��Y��Z | |
| D�� | ��YΪNa����X��Z��������ͬһ����Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
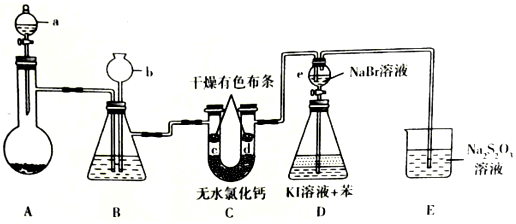
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�33.6L����������ˮ��Ӧ��ת�Ƶ�����ĿΪ1.5NA | |
| B�� | 20gH218O�к��е�������Ϊ10NA | |
| C�� | 12g���ʯ�к��еĹ��ۼ���Ϊ2NA | |
| D�� | ��״���£�33.6L�������к��з�ԭ�ӵ���Ŀ����1.5NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���³�ѹ�£�2.24 LSO2��������ԭ����Ϊ0.2NA | |
| B�� | ��1 mol Cl2ͨ��ˮ�У�HC1O��Cl-��ClO-������֮��Ϊ2NA | |
| C�� | 1 mol NO2������H2O��Ӧ��ת�Ƶĵ�����ΪNA | |
| D�� | 0.1 mol���ڵ�NaHSO4����������ĿΪ0��lNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
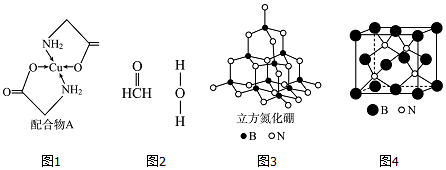
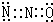 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
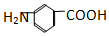 ��
��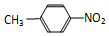 C��12C60��ʯī D���Ҵ����Ҷ��� E��35Cl��37Cl
C��12C60��ʯī D���Ҵ����Ҷ��� E��35Cl��37Cl�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com