
�ߴ� ���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������
���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������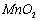 Ϊԭ���Ʊ������ߴ�
Ϊԭ���Ʊ������ߴ� �IJ����������£���1���Ʊ�
�IJ����������£���1���Ʊ� ��Һ��
��Һ��
����ƿ�У�װ�ü���ͼ������һ����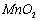 ��ˮ�����裬ͨ��
��ˮ�����裬ͨ��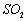 ��
�� ������壬��Ӧ3h��ֹͣͨ��
������壬��Ӧ3h��ֹͣͨ��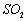 ��������ӦƬ�̣����ˣ���֪
��������ӦƬ�̣����ˣ���֪ ����
����
��ʯ������뷴Ӧ�Ļ�ѧ����ʽΪ ��

 �ڷ�Ӧ�����У�Ϊʹ
�ڷ�Ӧ�����У�Ϊʹ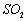 ������ת����ȫ����ͨ��
������ת����ȫ����ͨ��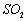 ��
�� ����һ�������ı��ҺͶ�ϵ������£��ɲ�ȡ�ĺ�����ʩ�� �� ��
����һ�������ı��ҺͶ�ϵ������£��ɲ�ȡ�ĺ�����ʩ�� �� ��
����ʵ���н� ���ɿ�������÷�ӦҺ��
���ɿ�������÷�ӦҺ��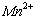 ��
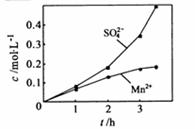
�� ��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��
��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��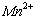 ��
�� Ũ�ȱ仯�������Բ����ԭ���� ��
Ũ�ȱ仯�������Բ����ԭ���� ��
��2���Ʊ��ߴ� ���壺��֪
���壺��֪ ������ˮ���Ҵ�����ʪʱ�ױ�����������100�濪ʼ�ֽ⣻
������ˮ���Ҵ�����ʪʱ�ױ�����������100�濪ʼ�ֽ⣻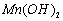 ��ʼ����ʱ
��ʼ����ʱ ���벹���ɣ�1���Ƶõ�
���벹���ɣ�1���Ƶõ� ��Һ�Ʊ��ߴ�
��Һ�Ʊ��ߴ� �IJ�������[ʵ���п�ѡ�õ��Լ���
�IJ�������[ʵ���п�ѡ�õ��Լ���  ��
�� ��
��  ��
�� ]��
]��
�� ���� ���� ���� ���ݵ���100����
���𰸡���1��
��Ca(OH)2+SO2=CaSO3+H2O
�ڿ����ʵ����¶� ����ͨ��������
��Mn2+��O2��H2SO3,��Ӧ����H2S04
��2��
�ٱ߽������NaH CO3(Na2CO3),��������ҺPH<7.7
CO3(Na2CO3),��������ҺPH<7.7
�ڹ��ˣ�������ˮϴ��2~3�Ρ�
�ۼ���SO42-�Ƿ�ϴ�ӳ�ȥ
��������C2H5OHϴ��(�������𰸾���)
��������������Ҫ�������ʵ��Ļ���������ʵ��������ѡ��ʵ�������������ʵ������Ŀ�����Ȼ���Ժ�ѧϰ��ϰ���ص㡣��1����ʯ������뷴Ӧ��Ҫ����SO2�ķ�Ӧ����Ϊ��SO2����ת�����ڱ������������£�����ͨ�������¶Ȼ���������������ȷ����Ӧ�ij�ֽ��У��۴�ͼ�п��Կ�����c(Mn2+)���٣�c(SO42-)���ӣ�������ΪMn2+��O2��H2SO3��Ӧ�����˴����ã���2�����������Ϣ�����Եó��Ʊ��ߴ�MnCO3�IJ������̣�����NaHCO3�ܽ⣬ͬʱ������Һ��pH<7.7�����ˡ�ϴ�ӡ�����SO42-�Ƿ�ȥ��Ϊ�˽���MnCO3����ģ���C2H5OHϴ�ӡ����¸��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������͵����������dz��õĻ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��
(1)����������SO2����������SO3��
2SO2(s)+O2��g�� 2SO3��g��.
2SO3��g��.
ij�¶��£�SO2��ƽ��ת����(��)����ϵ��ѹǿ(P)�Ĺ�ϵ����ͼ��ʾ������ͼʾ�ش��������⣺
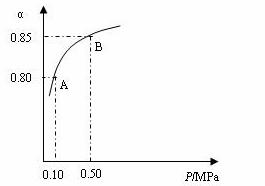
�ٽ�2.0 molSO2��1.0molO2����10 L�ܱ������У���Ӧ��ƽ�����ϵ��ѹǿΪ0.10MPa���÷�Ӧ��ƽ�ⳣ������__________��
��ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��K��A��_______K(B)(���������������=��)��
(2)��CH4����ԭNOx�������������������Ⱦ�����磺
CH4(g)+4NO2(g)=4NO(g)+CO2(g)+2H2O(g) ��H=-574kJ��mol-1
CH4(g)+4NO(g)=2N2(g)+CO2(g)+2H2O(g) ��H=-1160kJ��mol-1
���ñ�״����4.48 L CH4��ԭNO2��N2������������ת�Ƶĵ�������Ϊ__________(�����ӵ�������ֵ��NA��ʾ)���ų�������Ϊ___________kJ��
(3)�������ײ�����ȱλ������(MFe2Ox��3��x��4��M=Mn��Co��Zn��Ni)��������(MFe2O4)�����»�ԭ���ã������£�����ʹ��ҵ�����е�����������ֽ��ȥ��ת��������ͼ��ʾ��
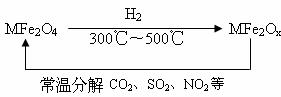
��д��MFe2Ox�ֽ�SO2�Ļ�ѧ����ʽ________________(������ƽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ�������Ԥ��ʵ��Ŀ�Ļ�����ʵ�����һ�µ���
| ѡ�� | ʵ����� | ʵ��Ŀ�Ļ���� |
| A | ij�����������ᣬ������ʹ����ʯ��ˮ����ǵ���ɫ��ζ���� | ˵���ü�����K2CO3 |
| B | ��������FeCl3��MgCl2��Һ�м�������Mg(OH)2��ĩ������һ��ʱ������ | ��ȥMgCl2��Һ������FeCl3 |
| C | �����£���Na2CO3��Һ�м�����BaSO4��ĩ�����ˣ���ϴ���ij����м�ϡ���ᣬ�����ݲ��� | ˵��������K sp(BaCO3)��K sp(BaSO3) |
| D | C2H5OH��Ũ����170�湲�ȣ��Ƶõ�����ͨ������KMnO4��Һ | �����Ƶõ������Ƿ�Ϊ��ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ�������Ԥ��ʵ��Ŀ�Ļ�����ʵ�����һ�µ���
| ѡ�� | ʵ����� | ʵ��Ŀ�Ļ���� |
| A | ij�����������ᣬ������ʹ����ʯ��ˮ����ǵ���ɫ��ζ���� | ˵���ü����� |
| B | �������� | ��ȥ |
| C | �����£��� | ˵��������
|
| D |
| �����Ƶ������Ƿ�Ϊ��ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ӵ�صĹ㷺Ӧ��ʹ��������﮻� Դ��Ϊ��Ҫ���⣺ij�о���ѧϰС��ԷϾ�����ӵ���������ϣ�LiMn2O4��̼�۵�Ϳ���������ϣ�������Դ�����о������ʵ���������£�
Դ��Ϊ��Ҫ���⣺ij�о���ѧϰС��ԷϾ�����ӵ���������ϣ�LiMn2O4��̼�۵�Ϳ���������ϣ�������Դ�����о������ʵ���������£�
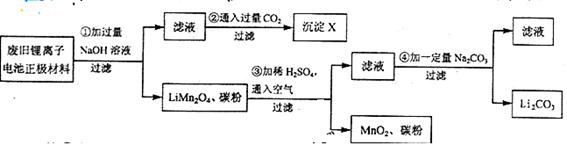
��1���ڢڲ���Ӧ�õ��ij���X�Ļ�ѧʽΪ ��
��2���ڢ۲���Ӧ�����ӷ���ʽ�� ��
��3���ڢܲ���Ӧ����Li2CO3����IJ��������� ��
������ʱ������Һ�����������ǣ���ʵ������ĽǶȸ������ֿ��ܵ�ԭ��
�� ��
��4�����Ͼ�����ӵ���������Ϻ�LiNB2O4������Ϊ18.1 g�ڢ۲���Ӧ�м���20.0mL3.0mol��L-1��H2SO4��Һ�������������е�﮾���Ӧ�ۺ͢���ȫΪLi2CO3,ʣ������ Na2CO3�μ��˷�Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��������ɰ�����в����Ĺ�����ϣ���Ҫ����MgCO3��MgSiO3��CaMg��CO3��2��Al2O3��Fe2O3�ȣ���������þ�Ĺ����������£�

| ������ | Fe��OH��3 | Al��OH��3 | Mg��OH��2 |
| pH | 3.2 | 5.2 | 12.4 |
������������ ����������ʽ��ȫ����ʱ��Һ��pH���ϱ�����ش��������⣺
����������ʽ��ȫ����ʱ��Һ��pH���ϱ�����ش��������⣺
��1���������������У�Ϊ���þ�Ľ����ʣ��ɲ�ȡ�Ĵ�ʩ��________________________��Ҫ��д����������
��2�����������Ҫ�ɷ���____________________________��
��3������Һ���пɻ������õ���Ҫ������________________________��
��4��Mg��ClO3��2��ũҵ�Ͽ�������Ҷ������������ɲ��ø��ֽⷴӦ�Ʊ���
MgCl2+2NaClO3====Mg��ClO3��2+2NaCl
��֪���ֻ�������ܽ�ȣ�S�����¶ȣ�T���仯��������ͼ��ʾ��
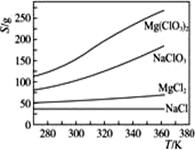
�ٽ���Ӧ�ﰴ��ѧ��Ӧ����ʽ�������Ȼ���Ʊ�Mg��ClO3��2���������Ʊ�Mg��ClO3��2��ԭ��________________________________________________________________________��
�ڰ��������������Ʊ�ʵ�顣����ȴ��������Mg��ClO3��2�����У�������NaCl������ԭ���ǣ�______________________________����ȥ��Ʒ�и����ʵķ����ǣ�_______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijʵ��С����������װ�ã����̶ֹ�װ���ԣ��Ʊ������ƣ�Ca3N2������̽����ʵ��ʽ��

��1����ͼ���Ӻ�ʵ��װ�á����װ�õ������ԣ�������_____________________
______________________________________________________________________��
��2����Ӧ������ĩ�˵��ܱ���ʼ�ղ����Թ�A��ˮ�У�Ŀ����_______________________
_______________________________________��
��3���Ʊ������ƵIJ��������ǣ��ٴ���K��ͨ��N2���ڵ�ȼ�ƾ��ƣ����з�Ӧ���۷�Ӧ������__________________���ܲ��װ�ã�ȡ�����
��4�����ݼ�¼���£�
| �մ�������m0/g | ������Ƶ�����m1/g | ��������������m2/g |
| 14.80 | 15.08 | 15.15 |
�ټ���õ�ʵ��ʽCaxN2������x��_______________________��
����ͨ���N2�л�������O2����Ƚ�x��3�Ĵ�С���������ж����ݣ�_________________
___________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ע�⣺����Ϊ�ֲ��⣬��A��B���⣬��������ѡһ�⡣�����������һ�ɰ�A��Ʒ֡�A���ʺ�ʹ�ö��ڿθ��½̲ĵĿ������B���ʺ�ʹ��һ�ڿθĽ̲ĵĿ������
��A������ͼ��ʾ�����ס�������װ�в�ͬ���ʵ���Ͳ�õ�������������������Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ�ͬʵ�飨������ͬ��ͬѹ�²ⶨ����

| ʵ����� | ����Ͳ������ | ����Ͳ������ | ����Ͳ������ |
| 1 | 10 mL FeSO4��Һ | 10 mL NH3 | ���ɰ�ɫ���������ɫ |
| 2 | 20 mL H2S | 10 mL SO2 | |
| 3 | 30 mL NO2����Ҫ�� | 10 mL H2O(l) | ʣ����ɫ���壬�����Զ�����ѹ�� |
| 4 | 15 mL Cl2 | 40 mL NH3 |
�Իش��������⣺
��1��ʵ��1�У��������ձ�Ϊ___________ɫ��д��������ɫ�Ļ�ѧ����ʽ_______________��
��2��ʵ��2����Ͳ�ڵ������ǣ���________���ɣ�����___________�ƶ�������⡱�����ڡ�����������Ӧ�����Ͳ���������IJ������壬��ȷ�Ĵ��������ǽ���ͨ��__________��Һ�С�
��3��ʵ��3�У����е�30 mL������NO2��N2O4�Ļ�����壬��ô�������ʣ�����ɫ������__________��д��NO2��H2O��Ӧ�Ļ�ѧ����ʽ_______________________________��
��4��ʵ��4�У���֪��3Cl2+2NH3 N2+6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ�仯Ϊ___________�������Ͳ��ʣ����������ԼΪ______________mL��
N2+6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ�仯Ϊ___________�������Ͳ��ʣ����������ԼΪ______________mL��
��B��ijʵ��С��������װ�ý����Ҵ���������ʵ�顣

��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ��Ӧ����ʽ
_____________________________________________________________________
_____________________________________________________________________��
�ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�������Ӧ��________��Ӧ��
��2����������ˮԡ���ò���ͬ��
��������____________________���ҵ�������_____________________��
��3����Ӧ����һ��ʱ������Թ�a�����ռ�����ͬ�����ʣ�������____________________________������ƿ���ռ������������Ҫ�ɷ���______________��
��4�����Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����__________��Ҫ��ȥ�����ʣ������ڻ��Һ�м���______________����д��ĸ����
a.�Ȼ�����Һ  b.��
b.��
c.̼��������Һ d.���Ȼ�̼
Ȼ����ͨ��_____________����ʵ��������ƣ����ɳ�ȥ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͭ�ж�����;��������������ɫ��������������ȡ�Ϊ��ô���������ͭ��̽�������ʣ�ijͬѧ�ù�ҵ����ͭ(����������������)��������ʵ�飺
���Ʊ�����ͭ
��ҵCuSO4
 CuSO4��Һ
CuSO4��Һ CuSO4��5H2O��������CuO
CuSO4��5H2O��������CuO
�ٲ���I��Ŀ���dz����������ʡ������� ��
�ڲ�����Ŀ���dz����������ǣ��μ�H2O2��Һ���Լ��ȣ���Fe2+ת����ȫ����������Cu2(OH)2CO3��ĩ�����裬�Կ�����ҺpH=3.5���������һ��ʱ�䣬���ˣ���ϡ�����ữ��Һ��pH=1��������ҺpH=3.5��ԭ���� ��
�۲�����Ŀ���ǵõ�CuSO4��5H2O���塣������ �����ˣ�ˮԡ���Ⱥ�ɡ�ˮԡ���ȵ��ص��� ��
��̽������ͭ������
��ȡA��B��֧�Թܣ���A���ȼ�������CuO��ĩ���ٷֱ���A��B�м���������3% H2O2��Һ��ֻ�۲쵽A���д������ݡ������� ��
��Ϊ̽���Թ�A�з�Ӧ�����ʣ��ռ����岢�ⶨ����������ʵ�������У�
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com