
| A������һ�����ʵ���Ũ�ȵ���Һʱ������ˮ��������ƿ�Ŀ̶��ߣ��ý�ͷ�ιܽ�����Һ���������� |
| B���ýྻ�IJ�˿պȡ������Һ�ھƾ��ƻ��������գ�����ʻ�ɫ������Һ��һ��������K+ |
| C������FeCl3��Һ��Fe��OH��3����ʱ���ɽ����Ƿֱ���һ�������䣬���������ЧӦ���� |
| D������ij��Һ���Ƿ���Fe3+ʱ�����ȼ�����������ˮ���ٵμ����軯����Һ������Һ��Ϊ��ɫ����˵����Һ��һ������Fe3+ |


 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
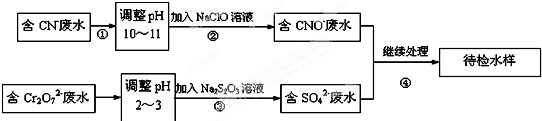
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ܱ�������ͨ��ˮ���� |
| B������ |
| C������������䣬���뺤��ʹ��ϵѹǿ���� |
| D������ѹǿ���䣬���뺤��ʹ����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Mg2+��Ca2+��HCO3-��CI- |
| B��Na+��CO32-��Cl-��SO42- |
| C��K+��Fe2+��SO42-��Br- |
| D��Fe2+��Ca2+��Cl-��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������0.4 mol/L HB��Һ��0.2 mol/L NaOH��Һ�������Ϻ���Һ��pH=3��������Һ������Ũ�ȵĴ�С˳��Ϊ��c��Na+����c��B-����c��H+����c��OH-�� |
| B������ʱ��pH=2��CH3COOH��Һ��HCl��Һ��pH=12�İ�ˮ��NaOH��Һ��������Һ����ˮ�����c��H+������� |
| C��pH=11��NaOH��Һ��pH=3�Ĵ�����Һ�������ϣ�����ʯ����Һ�ʺ�ɫ |
| D��0��lmol/L pHΪ4��NaHB��Һ�У�c��HB-����c��H2B����c��B2-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ʣ�࣬��Һ��dz��ɫ��Cl-Ũ�Ȼ������� |
| B������Һ�е���KSCN��ɫ��Һ�����Ժ�ɫ |
| C�����������뻹ԭ��������ʵ���֮��Ϊ2��5 |
| D��Fe2+��Fe3+�����ʵ���֮��Ϊ6��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
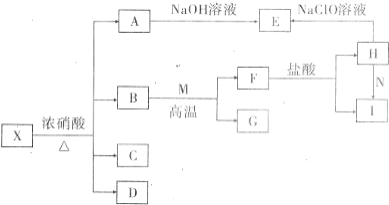 ����X�������ֳ���Ԫ����ɵĻ�������н���Ԫ������һԪ�ص�������Ϊ14��1����һ��������X�ɷ�������ת�������ֲ���δ�������C����ɫ��������ʹ����ʯ��ˮ����ǵ����壬DΪ����ɫ���壬EΪ���ɫ������MΪ�����������ʣ�
����X�������ֳ���Ԫ����ɵĻ�������н���Ԫ������һԪ�ص�������Ϊ14��1����һ��������X�ɷ�������ת�������ֲ���δ�������C����ɫ��������ʹ����ʯ��ˮ����ǵ����壬DΪ����ɫ���壬EΪ���ɫ������MΪ�����������ʣ��鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com