�Ͼ���Ļ������ü������ڽ�Լ��Դ���������ڱ���������ij�о�С��ͬѧ�ԷϾ�п�̸ɵ��Ϊԭ�ϣ����Ͼɵ�غ�п����ת����ZnSO
4?7H
2O�����̲���ת���ɴ��Ƚϸߵ�MnO
2����NH
4Cl��ҺӦ���ڻ��������У�ʵ���������£�
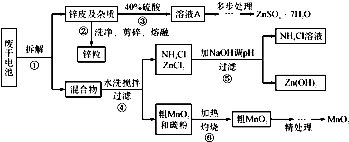
��1�������������õļ�������Ӧѡ
����
����
�������������������
��2������ҺA�����ĵ�һ���Ǽ��백ˮ����pHΪ9��ʹ���е�Fe
3+��Zn
2+ ��������д����ˮ��Fe
3+��Ӧ�����ӷ���ʽ��
Fe3++3NH3?H2O=Fe��OH��3��+3NH4+
Fe3++3NH3?H2O=Fe��OH��3��+3NH4+
��
��3����������Ϊ�˳�ȥ��Һ�е�Zn
2+����֪25��ʱ��һЩ���ݼ��±���
| NH3?H2O��Kb |
Zn 2+��ȫ������pH |
Zn��OH��2���ڼ��pH |
| 1.8��10-5 |
8.9 |
��11 |
���ϱ����ݷ���Ӧ������ҺpH���Ϊ
a
a
������ĸ����
a.9����������b.10����������c.11
��4��MnO
2����������Ҫ���裺
����1����3%H
2O
2��6.0mol?L
-1��H
2SO
4�Ļ��Һ����MnO
2�ܽ⣬���ȳ�ȥ����H
2O
2����MnSO
4��Һ��������Fe
3+ ������Ӧ����MnSO
4�����ӷ���ʽΪ
MnO2+H2O2+2H+=Mn2++2H2O+O2��
MnO2+H2O2+2H+=Mn2++2H2O+O2��
��
����2����ȴ�����£��μ�10%��ˮ����pHΪ6��ʹFe
3+ ������ȫ���ټӻ���̿���裬���ˣ��ӻ���̿��������
�����۳������������������γɽϴ��������
�����۳������������������γɽϴ��������
��
����3������Һ�еμ�0.5mol?L
-1��Na
2CO
3��Һ������pH��7���˳�������ϴ�ӡ�������ڿ������������ں�ɫ������MnO
2�����չ����з�Ӧ�Ļ�ѧ����ʽΪ
��
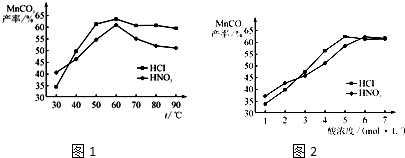
��5��������֪����MnO
2���ܽ�������������������ݣ�Ȼ����ȡMnCO
3���壮
���������������Һ��Ũ�Ⱦ�Ϊ5mol?L
-1�������Ⱥ���ѽ���ʱ���£������¶ȶ�MnCO
3���ʵ�Ӱ����ͼ1����ͼ�������������ѽ����¶ȶ���
60
60
�����ң�
��������¶ȡ���ѽ���ʱ����������£����Ũ�ȶ�MnCO
3���ʵ�Ӱ����ͼ2����ͼ������������Ũ��Ӧѡ��
6
6
mol?L
-1���ң�



 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
 ��2013?������ģ����ҵ�ϡ��̶���������CO2����Ч�ؼ��ᡰ����ЧӦ����
��2013?������ģ����ҵ�ϡ��̶���������CO2����Ч�ؼ��ᡰ����ЧӦ����
