
����Ŀ������CO��H2�ڴ����������ºϳɼ״��������ķ�Ӧ���£�CO(g)+2H2(g)![]() CH3OH(g)�������һ�����ܱ������а����ʵ���֮��1��2����CO��H2�����ƽ��������CH3OH����������ڲ�ͬѹǿ�����¶ȵı仯��ͼ��ʾ������˵����ȷ����
CH3OH(g)�������һ�����ܱ������а����ʵ���֮��1��2����CO��H2�����ƽ��������CH3OH����������ڲ�ͬѹǿ�����¶ȵı仯��ͼ��ʾ������˵����ȷ����

A. �÷�Ӧ����H��0����p1��p2
B. ��Ӧ���ʣ�����(״̬A)������(״̬B)
C. ��C��ʱ��COת����Ϊ75%
D. �ں��º�ѹ���������ܱ������г��벻ͬ����CH3OH����ƽ��ʱCH3OH���������Ҳ��ͬ
���𰸡�C
�����������������A����ͼ��֪�������¶ȣ�CH3OH�����������С��ƽ�������ƶ�����÷�Ӧ����H��0��300��ʱ������ѹǿ��ƽ�������ƶ���CH3OH�����������������p1��p2����A����B��B���Ӧ���¶Ⱥ�ѹǿ������A�㣬�¶����ߡ�����ѹǿ��ʹ�÷�Ӧ�Ļ�ѧ��Ӧ���ʼӿ죬���������״̬A����������״̬B������B����C�������ܱ�����������1molCO��2molH2��CO��ת����Ϊx����
CO��g��+2H2��g��![]() CH3OH��g��
CH3OH��g��
��ʼ 1 2 0
�仯 x 2x x
���� 1-x 2-2x x
��C��ʱ��CH3OH���������=![]() =0.5�����x=0.75����C��ȷ��D���ɵ�Чƽ���֪���ں��º�ѹ���������ܱ������г��벻ͬ����CH3OH����ƽ��ʱCH3OH�������������ͬ����D����ѡC��
=0.5�����x=0.75����C��ȷ��D���ɵ�Чƽ���֪���ں��º�ѹ���������ܱ������г��벻ͬ����CH3OH����ƽ��ʱCH3OH�������������ͬ����D����ѡC��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Һһ�������Ե���
A. pH��7����Һ B. c(H+)��c(OH��) ����Һ
C. pH��7����Һ D. c(H+)��c(OH��) ����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���мס��ҡ���������Һ��������KCl��NaBr��KI������м��������Һ����ˮ����Һ��Ϊ��ɫ���ټ��������ɫ�����Ա仯����ס��ҡ������κ��У�������
A.NaBr��KCl��KI
B.NaBr��KI��KCl
C.KI��NaBr��KCl
D.KCl��KI��NaBr
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����þ�Ͻ��ѳ�Ϊ�ִ����졢������������ҵ����Ҫ���ϡ�ij�о���ѧϰС�����λͬѧ��Ϊ�ⶨij��þ3%��5%����þ�Ͻ�(��������Ԫ��)��þ����������������������ֲ�ͬʵ�鷽������̽������д���пհס�
̽��һ
ʵ�鷽������þ�Ͻ�![]() �ⶨʣ�����������ʵ���з�����Ӧ�Ļ�ѧ����ʽ��_______________________________________��
�ⶨʣ�����������ʵ���з�����Ӧ�Ļ�ѧ����ʽ��_______________________________________��
ʵ�鲽�裺
(1)��ȡ5.4 g��þ�Ͻ��ĩ��Ʒ��Ͷ��V mL 2.0 mol/L NaOH��Һ�У���ַ�Ӧ��Ϊ��֤�Ͻ��ַ�Ӧ��NaOH��Һ�����V��________��

(2)���ˡ�ϴ�ӡ�����������塣�ò�������δϴ�ӹ��壬���þ������������________(����ƫ��������ƫ����)��
̽����
ʵ�鷽������þ�Ͻ�![]() �ⶨ������������(ʵ��װ����ͼ��ʾ��)���������ۣ�
�ⶨ������������(ʵ��װ����ͼ��ʾ��)���������ۣ�
(1)ijͬѧ�����ʵ��װ�ò������ƣ�Ӧ��A��B֮������һ�������������װ�á���������________(������Ҫ����������Ҫ��)��
(2)Ϊʹ�ⶨ��������ܾ�ȷ��ʵ����Ӧע���������(д������)��
��______________________________________��
��______________________________________��
̽����
ʵ�鷽��������x g��þ�Ͻ��ĩ������װ�����������������ж��Ե��Ȱ��ϣ�ͨ��ʹ�������ա�
�������ۣ�
(1)������Mg��������������ʵ���л���ⶨ��������__________________��
(2)���ÿ�������O2����ʵ�飬�Բⶨ����Ƿ���Ӱ�죿________(����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��DΪ���ֿ����Ե��Σ����ǵ������ӷֱ������Ba2+��Ag+��Na+��Cu2+�е�ijһ�֣������ӷֱ������![]() ��
��![]() ��Cl��
��Cl��![]() �е�һ�֡�(�����������в��ظ�����)
�е�һ�֡�(�����������в��ظ�����)
�����������ηֱ�����ʢ������ˮ����֧�Թ��У�ֻ��C�ε���Һ����ɫ��
������ٵ���֧�Թ��зֱ�������ᣬB�ε���Һ�г������ɣ�D�ε���Һ����ɫ��ζ�������ݳ���
���ݢ٢�ʵ����ʵ���ƶϣ�
(1)A�Ļ�ѧʽΪ____________��B�Ļ�ѧʽΪ____________��
(2)д������������D��Ӧ�����ӷ���ʽ��____________��
(3)д��C��Ba(OH)2��Һ��Ӧ�����ӷ���ʽ��________________________��
(4)C��Һ�������ӵļ��鷽����_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ҫ����������Ʒ֮һ���ڹ�����ռ����Ҫ��λ���ҹ���������ý(����Ϊ���Ļ����)�������ϳɰ����ش�����������
��1��Fe��̬ԭ�Ӻ�������Ų�ʽΪ____����������һ����Ҫ���������������ˮ����¶�ڳ�ʪ�����п��ͷų�������Ԫ��Fe��N�У���һ�����ܽϴ����_____����̬ԭ�Ӻ���δ�ɶԵ������϶����_______��
��2��N����������N2O����N2O��CO2��Ϊ�ȵ����壬N2O�Ŀռ乹��Ϊ__________��
��3��N�ж����⻯�������(N2H4)����������ƽ�����ȼ�ϣ�N2H4��Nԭ�ӵ��ӻ���ʽΪ____��
��4��N��P��AsΪͬ��Ԫ�أ�NH3��PH3��AsH3�������ʵķе��ɸߵ��͵�˳��Ϊ_____��ԭ����____��
��5��K3[Fe(CN)6]�����ڼ���Fe2����K3[Fe(CN)6]�д��ڵĻ�ѧ��������_______��
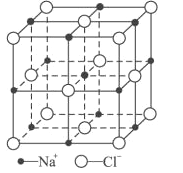
��6��FeO����ľ�����NaCl�����ƣ�NaCl�ľ�����ͼ��ʾ�����ھ���ȱ�ݣ�ij�������������ʵ�����ΪFe0.9O�����а�����Fe2����Fe3���������߳�Ϊ428pm����þ�����ܶ�Ϊ____g/cm3(�г�����ʽ���ɣ���NA��ʾ�����ӵ�������ֵ)��
 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����С�����þ��CO2�ķ�Ӧ���ʵ��̽��þ��NO2�ķ�Ӧ��
��ͬѧ�Ʋ������MgO��N2��
��ͬѧ�Ʋ�������MgO��N2�⣬�����л����ܺ���Y��
��С��ͬѧ���������װ��̽��þ��NO2��Ӧ�Ĺ��������ⶨ����ɡ�
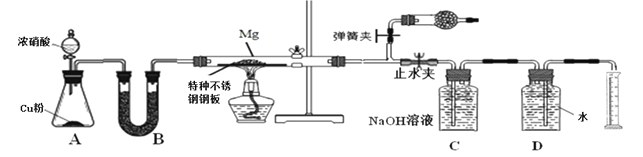
��1��ʵ�鿪ʼʱ���ȹر�ֹˮ�к���ɼУ��ٴ�Һ©����������Ӳ�ʲ����ܳ�������ɫ�����ֹˮ�У��رյ��ɼУ�����ȼ�ƾ��ơ���������Ŀ����___________________________________________________________
��2��װ��B�е��Լ�����ѡ��________
A��Ũ���� B����ˮ�Ȼ��� C������������ D����ʯ��
��3��װ��C��ʢװ����������Һ�������ǣ�___________________________________
��4��Ϊ��֤�������������ȷ�ԣ���ȡ��Ͳ�̶�ʱӦע��������Ǣ�����ָ��������ٶ�������_______________________________����______________________________��
��5��ʵ���������ͬѧ�ǽ��������ȡ����ˮ��Ӧ�������д̼�����ζ�������������������ʹʪ���ʯ����ֽ������˵������ͬѧ�Ʋ���ȷ����д��Y��ˮ��Ӧ�Ļ�ѧ����ʽ__________________________________
��6������ʼ����þ������Ϊ3.6 g����������NO2�г�ַ�Ӧ�� ���ռ���N2���Ϊ448mL (��״��)���������MgO��������_________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ɫ����N2O4��һ��ǿ��������Ϊ��Ҫ�Ļ���ƽ���֮һ��N2O4��NO2ת�����Ȼ�ѧ����ʽΪN2O4(g)![]() 2NO2(g)����H����24.4 kJ/mol��
2NO2(g)����H����24.4 kJ/mol��
(1)��һ����N2O4Ͷ��̶��ݻ�����������У�����������˵����Ӧ�ﵽƽ����� _________��
a��v��(N2O4)��2v��(NO2)
b����ϵ��ɫ����
c������ƽ����Է�����������
d�������ܶȲ���
�ﵽƽ�����������������¶ȣ��ٴε���ƽ��ʱ�����������ɫ____(�� ���������dz�����䡱)���ж�����_______��
(2)ƽ�ⳣ��K���÷�Ӧ��ϵ���������ʷ�ѹ��ʾ����K����ʽ����ƽ���ѹ����ƽ��Ũ�ȣ���ѹ����ѹ�����ʵ�������[���磺p(NO2)��p����x(NO2)]��д��������Ӧƽ�ⳣ��Kp����ʽ _______________________(��p�������������ʵ�������x��ʾ)��Ӱ��Kp������_________________��
(3)������Ӧ�У�����Ӧ����v����k����p(N2O4)���淴Ӧ����v����k����p2(NO2)������k����k��Ϊ���ʳ�������KpΪ____(��k����k����ʾ)������һ����N2O4Ͷ����������к��º�ѹ�ֽ�(�¶�298 K��ѹǿ100 kPa)����֪��������k����4.8��104 s��1����N2O4�ֽ�10%ʱ��v����______________kPa��s��1��
(4)����ܱ������з���һ����N2O4��ά����ѹǿp0�㶨�����¶�ΪTʱ��ƽ��ʱN2O4�ֽ�ٷ���Ϊ���������¶Ȳ��䣬���ܱ������г������N2O4��ά����ѹǿ��2p0�����·ֽ⣬��N2O4��ƽ��ֽ��ʵı���ʽΪ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ij�����Ħ������ΪM��һ�������µ�Ħ�����ΪVm �� ����˵����ȷ���ǣ�������
A.һ����������ӵ�����ΪM/NA
B.һ����������ӵ����ΪVm/NA
C.����Ħ������Ĵ�Сȡ����������ӱ����Ĵ�С
D.����˵��������ȷ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com