
�Ա���ۣ���Ҫ�ɷ�ΪBaCO3������Ca2+��Fe2+��Fe3+��Mg2+�ȣ��Ʊ�BaCl2��2H2O���������£� 
��1������������Ҫ��Ӧ�����ӷ���ʽΪ ��
��2������C����Ҫ�ɷ���Ca(OH)2�� ����ͼ��֪��Ϊ�˸��õ�ʹCa2+��������Ӧ��ȡ�Ĵ�ʩΪ ��

��3����BaSO4�������ⶨ��Ʒ���ȵIJ���Ϊ��
����1��ȷ��ȡ0.4��0.6 g BaCl2��2H2O����������100 ml ˮ��3 ml 2 mol��L-1��HCl��Һ�����ܽ⡣
����2���߽��裬����μ���0.1 mol��L-1 H2SO4��Һ��
����3����BaSO4������ ��ȷ������ȫ������
����4�����ˣ���0.01 mol��L-1��ϡH2SO4ϴ�ӳ���3~4�Σ�ֱ��ϴ��Һ�в���Cl��Ϊֹ��
����5�����۵��ij�����ֽ������ �У�����ɡ�̿�����һ�����800�����������ء���������BaCl2��2H2O��Ba2+�ĺ�����
�ٲ���3��ȱ�IJ���Ϊ ��
��������1��������Ʒ���٣����ڲ���4ϴ��ʱ������ɵ�Ӱ��Ϊ ��
�۲���5���ô�����������Ϊ ����ֽ�һ�ʱ����Ҫ���㣬����BaSO4�ױ�������̿��ԭ����BaS���÷�Ӧ�Ļ�ѧ����ʽΪ ��
����ͬѧ��Ϊ��K2CrO4����H2SO4��������Ч�����ã���˵��ԭ�� ��
[��֪��Ksp(BaSO4)=1.1��10-10 Ksp(BaCrO4)=1.2��10-10]
��1��H2O2+ 2Fe2++2H+ =2Fe3+ +2H2O
��2��Mg(OH)2 ����¶�
��3�������ϲ���Һ�м���1~2��0.1mol/LH2SO4��Һ
�ڳ�ȡ�������٣����������٣�ϴ����ɵ���ʧ�ʹ�
������ BaSO4 + 4C 4CO + BaS��BaSO4 + 2C
4CO + BaS��BaSO4 + 2C 2CO2 + BaS
2CO2 + BaS
��BaCrO4��Ħ����������BaSO4
�����������������Ϊ���������⣬����Ҫ�ɷ�ΪBaCO3����ۣ�������ȥ����Ca2+��Fe2+��Fe3+��Mg2+�������Ʊ�BaCl2��2H2O�����̡���2������C֮ǰ�ѳ�ȥ�������Ըò��dz�ȥʣ�������Ca2+��Mg2+��������Ӧ���������ͼ������Ca(OH)2�ܽ�����¶����߶����ͣ��ʸ�����������Ca2+��������3���ٲ���3��Ȼ��ȷ�������Ƿ���ȫ�IJ�����Ӧ���Ǿ��ã������ϲ���Һ�м���1~2��ԭH2SO4��Һ���������ֳ�������Ba2+������ȫ���ڳ�ȡ�������٣����������٣�ϴ����ɵ���ʧ�ʹ۹�������һ�㶼�������У����������ʵ�Ksp������ͬ����BaCrO4��Ħ����������BaSO4��ʵ�������ɵij��������������С��
���㣺���������⣬�������ʵķ��롢���ӡ����顢ʵ������Ȼ���ʵ��������й����⡣


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ʽ̼��ͭ��Cu2(OH)2CO3����һ����;�㷺�Ļ���ԭ�ϣ�ʵ�����Է�ͭмΪԭ����ȡ��ʽ̼��ͭ�IJ������£�
����һ����ͭм������ͭ
��ͼ���ý�ͷ�ι���ȡŨHNO3�����ӵ���ƿ�ڵķ�ͭм��(��ͭм����)����ַ�Ӧ����ˣ��õ�����ͭ��Һ��
���������ʽ̼��ͭ���Ʊ�
����Թ��м���̼������Һ������ͭ��Һ��ˮԡ������70�����ң���0��4 mol��L��NaOH��Һ����pH��8��5�������ã����ˣ�����ˮϴ�ӣ���ɣ��õ���ʽ̼��ͭ��Ʒ��
���������գ�
��1��д��Ũ������ͭ��Ӧ�����ӷ���ʽ�� ��
��2����ͼװ����NaOH��Һ��������_ ����Ӧ�������ƿ�ڵ���Һ�У����˺���NaOH�⣬����___ _(��д��ѧʽ)��
��3��������У�ˮԡ��������������__ _��_ (���ȡ��г�������ʯ��������)��
��4�����������Һ�п��ܺ���CO32-��д������CO32-�ķ�����
��5��Ӱ���Ʒ��������Ҫ������__ __��
��6����ʵ��õ�2��42 g��Ʒ(ֻ��CuO����)��ȡ����Ʒ�������ֽ���ȫ�õ�1��80 g���壬����Ʒ�м�ʽ̼��ͭ������������___ _��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
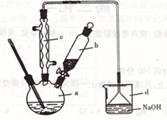
ʵ�����Ʊ������飨C2H5Br����װ�úͲ�����ͼ������֪������ķе�38.4�棩
�ټ��װ�õ������ԣ���װ��ͼ��ʾ��U�ܺʹ��ձ��м����ˮ��
����Բ����ƿ�м���10mL95%�Ҵ���28mLŨ���ᣬȻ�������ϸ��13g�廯�ƺͼ������Ƭ��
��С����ȣ�ʹ���ַ�Ӧ��
�ش��������⣺
��1����ʵ����ȡ������Ļ�ѧ����ʽΪ�����ɵ���ΪNaHSO4����______________________��
��2����Ӧʱ���¶ȹ��ߣ��ɿ����к���ɫ��������������廯ѧʽΪ__________��
��3��Ϊ�˸��õĿ��Ʒ�Ӧ�¶ȣ�����ͼʾ��С����ȣ����õļ��ȷ�ʽ��__________��
��4��U���ڿɹ۲쵽��������_____________________________��
��5����Ӧ������U�ι��д��Ƶ�C2H5Br���ػ�ɫ��Ϊ�˳�ȥ�ֲ�Ʒ�е����ʣ���ѡ�������Լ��е�_________________������ţ�
a���� b��H2O c��Na2SO3��Һ d��CCl4
�������Ҫ����������______________�����������ƣ���
��6�����м���ʵ�鲽�裬�����ڼ�������������Ԫ�أ�����ȷ�IJ���˳���ǣ�ȡ���������飬Ȼ��__________________������ţ���
�ټ��ȣ��ڼ���AgNO3��Һ���ۼ���ϡHNO3�ữ���ܼ���NaOH��Һ������ȴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������ʵ�������Ԥ��ʵ��Ŀ�Ļ�����ʵ����ۣ���ȷ����̣�����������
| ѡ�� | ʵ����� | ʵ��Ŀ�Ļ���� | �ж� |
| �� | ij�����������ᣬ������ʹ����ʯ��ˮ����ǵ���ɫ��ζ���� | ˵���ü����� | |
| �� | ��ij��Һ�м������ᣬ�����������������м���BaCl2��Һ�а�ɫ���������� | ֤������Һ���� SO42�� | |
| �� | �������Һ�м���һ������ϡ������ȣ��ټ���һ����������������ͭ���ȡ� | ֤������ˮ����������� | |
| �� |  ��Ũ����170�湲�ȣ��Ƶõ�����ͨ������ ��Ũ����170�湲�ȣ��Ƶõ�����ͨ������ ��Һ ��Һ | �����Ƶ������Ƿ�Ϊ��ϩ | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������ȼҵ�еķ�������Ҫ����þ�����������ƵȵĹ����κ�̼���Ρ�ʵ����������Ϊԭ����ȡMgSO4��7H2O���������£�
��֪��(��) Ksp[Mg(OH)2]��6.0��
(��) Fe2+��Fe3+��Al3+��ʼ��������ȫ������pH��Χ����Ϊ��7.1~9.6��2.0~3.7��3.1~4.7
(��) ���ֻ�������ܽ�ȣ�S�����¶ȱ仯������ͼ��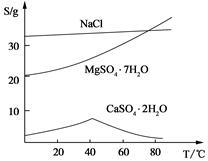
�ش��������⣺
��1���������м�H2SO4��Һ����pHΪ1��2�Լ���һ����е�Ŀ���ǣ�������
��2������Һ��Mg2+��Ũ��Ϊ6 mol/L����ҺpH�������ſ��ܲ���Mg(OH)2������
��3���ڶ��ι�����Ҫ���Ƚ��У���Ҫԭ����������������������Ҫ�ɷ����� ��
��4������Һ���л��MgSO4��7H2O�����ʵ���������Ϊ��������Һ���м����������ڹ��ˣ��ó�������������������Ũ�������½ᾧ���ݹ��ˡ�ϴ�ӵò�Ʒ��
��5������õ�MgSO4��7H2O����Ϊ24.6 g����������к�þ[��Mg(OH)2��]�İٷֺ���Լ ��MgSO4��7H2Oʽ��Ϊ246��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijͬѧ�ӱ������˽�����и��������Բ����κ�̼���Σ�������ʵ����֤��һ��ʵ���������κ�������һ���������ϵ�֪�����ᣨH2C2O4��������ǿ�ڴ���Ķ�Ԫ�л��ᣬ����һ�ֻ�ԭ�Խ�ǿ�����ʣ���2KMnO4+5H2C2O4+3H2SO4=2MnSO4+K2SO4+10CO2��+8H2O������ƣ�CaC2O4��������ˮ�ʹ��ᣬ������ǿ�CaC2O4+2H+= H2C2O4+Ca2+��
��1�����ʵ�鷽����֤�����к��в����κ�̼���Σ��������ʵ�鲽�衢Ԥ������ͽ��ۡ�
��ѡ�Լ���1 mol��L��1 H2SO4��1 mol��L��1 HCl��0.1 mol��L��1 NaOH��1 mol��L��1 CaCl2��0.01 mol��L��1 KMnO4������ʯ��ˮ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1����������ĥ���ݡ����˵õ����������Һ�� | |
| ����2��������Һ�������ԣ��μ�����CaCl2��Һ�� | ���ְ�ɫ������˵�������п��ܺ��в����κ�̼���Ρ� |
| ����3��ȡ����2�ij������Թ��У� | |
| ����4�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽��װ��ʾ��ͼ���й��������£�
| | �� | �� | �屽 |
| �ܶ�/g��cm-3 | 0. 88 | 3. 10 | 1. 50 |
| �е㣯�� | 80 | 59 | 156 |
| ˮ���ܽ�� | �� | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
δ�������н����ѵ�����Խ��Խ�㷺������ڴ���TiO2�����£�����NaClO��CN��(���Ժ�ǿ)������CNO�����������������¼�����NaClO������N2��CO2������������Ա���ܱ�ϵͳ������ͼװ�ý���ʵ�飬��֤��������������Ч�ԣ���ͨ���ⶨ������̼����ȷ��CN���������İٷ��ʡ�
��Ũ����CN�����ӵ���ˮ�����NaClO��Һ�Ļ��Һ��200mL������CN����Ũ��Ϊ0.05mol��L��1��������У�������Ƥ����һ��ʱ�����Ƥ���ͻ�����ʹ��Һȫ���������У��رջ������ش��������⣺
��1�����з�Ӧ�����ӷ���ʽΪ__________________________________��
��2���������ɵ������N2��CO2�⣬����HCl��������Cl2�ȣ�����ʵ����ͨ���ⶨ������̼������ȷ����CN���Ĵ���Ч��������м���ij����Լ���______������ĸ����
a������ʳ��ˮ b������NaHCO3��Һ c��ŨNaOH��Һ d��Ũ����
��3������ʵ���е�������____________________________��װ�м�ʯ�ҵĸ���ܵ�������____________________________��
��4������ʢ�к�Ca(OH)20.02mol��ʯ��ˮ����ʵ�������й�����0.82 g���������ʵ���в��CN���������İٷ��ʵ���________����˵���ò��ֵ��ʵ�ʴ����İٷ������ƫ����ƫ��_________����Ҫ˵�����ܵ�ԭ��________________________________________________________��
��5�������һ�������ȷ�ȵĽ��飨Ҫ�пɲ����ԣ�����ʹ������ù��ڸ��ӣ�
_________________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��������ȿ�����Ũ�������ֿ����ù����ʯ�Ҹ������
| A��SO2 | B��NH3 | C��Cl2 | D��H2 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com