
�������ȼҵ�еķ�������Ҫ����þ�����������ƵȵĹ����κ�̼���Ρ�ʵ����������Ϊԭ����ȡMgSO4��7H2O���������£�
��֪��(��) Ksp[Mg(OH)2]��6.0��
(��) Fe2+��Fe3+��Al3+��ʼ��������ȫ������pH��Χ����Ϊ��7.1~9.6��2.0~3.7��3.1~4.7
(��) ���ֻ�������ܽ�ȣ�S�����¶ȱ仯������ͼ��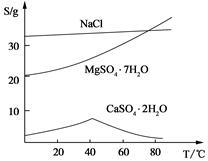
�ش��������⣺
��1���������м�H2SO4��Һ����pHΪ1��2�Լ���һ����е�Ŀ���ǣ�������
��2������Һ��Mg2+��Ũ��Ϊ6 mol/L����ҺpH�������ſ��ܲ���Mg(OH)2������
��3���ڶ��ι�����Ҫ���Ƚ��У���Ҫԭ����������������������Ҫ�ɷ����� ��
��4������Һ���л��MgSO4��7H2O�����ʵ���������Ϊ��������Һ���м����������ڹ��ˣ��ó�������������������Ũ�������½ᾧ���ݹ��ˡ�ϴ�ӵò�Ʒ��
��5������õ�MgSO4��7H2O����Ϊ24.6 g����������к�þ[��Mg(OH)2��]�İٷֺ���Լ ��MgSO4��7H2Oʽ��Ϊ246��
��15�֣�
��1�����Mg2+�Ľ�ȡ�ʣ�2�֣�
��2��8��2�֣�
��3���¶Ƚϸ�ʱ������þ�η�������ף��������CaSO4��2H2O�ܽ��С����2�֣�
Al(OH)3��Fe(OH)3��CaSO4��2H2O ��2�֣�
��4��NaOH��Һ��2�֣� ������м�����ϡ���ᣨ2�֣�
��5��20.0%��3�֣�
�������������������ͼ���٢ڢ�������ܽ⣬�ܷ�����Һ�������ʣ���Ҫ��H2SiO3�Ͳ��ܽ���������ݽ�Fe2�� ������Fe3�� �Ա�ֲ���ȥ������Fe (OH)3��Al (OH)3��MgSO4��NaCl��Һ������Һ��õ���Ʒ����ͨ�������߷�Ӧ���ʣ����Mg2�� �Ľ����ʣ��𰸣����Mg2+�Ľ�ȡ�ʣ���Ksp[Mg(OH)2]��6.0�� =c(Mg2��)c2(OH�D )�ã�c2(OH�D )= 6.0��
=c(Mg2��)c2(OH�D )�ã�c2(OH�D )= 6.0�� /6=1.0��10-12��c(OH�D )=1.0��10�D6mol/L,PH
/6=1.0��10-12��c(OH�D )=1.0��10�D6mol/L,PH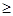 8���𰸣�8���Ǵ�ͼ�ж���������CaSO4��2H2O�ܽ��С������Һ��������ף��𰸣��¶Ƚϸ�ʱ������þ�η�������ף��������CaSO4��2H2O�ܽ��С���� Al(OH)3��Fe(OH)3��CaSO4��2H2O������Һ���к���MgSO4��NaCl��Ҫ��NaCl�����ȥ��Ҫ�Ƚ�Mg2�� �γ�Mg(OH)2���������˺�NaCl��ȥ��Ȼ���H2SO4��������MgSO4��Ȼ������Ũ�������½ᾧ�����ˡ�ϴ�ӡ�������þ��塣�𰸣�NaOH��Һ��������м�����ϡ�����MgSO4��7H2O����Ϊ24.6 g����0.1mol,��0.1molMg (OH)2Ϊ5.8 g��5.8g/29g��100%=20.0%���𰸣�20.0%��
8���𰸣�8���Ǵ�ͼ�ж���������CaSO4��2H2O�ܽ��С������Һ��������ף��𰸣��¶Ƚϸ�ʱ������þ�η�������ף��������CaSO4��2H2O�ܽ��С���� Al(OH)3��Fe(OH)3��CaSO4��2H2O������Һ���к���MgSO4��NaCl��Ҫ��NaCl�����ȥ��Ҫ�Ƚ�Mg2�� �γ�Mg(OH)2���������˺�NaCl��ȥ��Ȼ���H2SO4��������MgSO4��Ȼ������Ũ�������½ᾧ�����ˡ�ϴ�ӡ�������þ��塣�𰸣�NaOH��Һ��������м�����ϡ�����MgSO4��7H2O����Ϊ24.6 g����0.1mol,��0.1molMg (OH)2Ϊ5.8 g��5.8g/29g��100%=20.0%���𰸣�20.0%��
���㣺�ۺ�ʵ���⣬�漰Ԫ�ػ�����֪ʶ��������ԭ�ζ���ָʾ��ѡ����������ʵ�鲽�衢pH���ڵȶ�����ݣ�����ѧ�����ۺ�ʵ�鴦��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ȼ�����Ʒ�к�������̼��ء�����غͲ�����ˮ�����ʡ�Ϊ���ᴿ�Ȼ��أ��Ƚ���Ʒ��������ˮ�У���ֽ������ˣ��ٽ���Һ����ͼ��ʾ������в�����
(1)Ϊ������ʼ��Һ�е�Cl���������Լ���ϡHNO3����Ӧ��
(�ѧʽ)
(2)�Լ���Ļ�ѧʽ�ֱ�Ϊ �� ��
(3)����ٺ͢��У����õIJ�����������ͷ�ιܣ���Ӧ��
(4)������У��ж��Լ����Ѽ�������ķ����ǣ�
(5)ijͬѧ������������KCl�����ԭ��Ʒ������������Ȼ�����Ʒ�Ĵ��ȣ�����Ϊ�����Ƿ�ɿ�����˵���� (������ʵ�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���������ؾ��ں��зḻ��������ʳ����Դ��ʳ�����ճ������еı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
��1�����ⶨ���������´����к�������K+��Ca2+��Mg2+��Fe3+���������ӣ�ij�о���ѧϰС����ʵ�����ᴿNaCl���������£�
���ṩ���Լ�������Na2CO3��Һ������K2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ba(NO3)2��Һ��75%�Ҵ���Һ��CCl4����������Ʒ��ѡ��
������ȥ��Һ�е�Ca2+��Mg2+��Fe3+��SO42-��ѡ��a���������������Լ������μ�˳������Ϊ
��ֻ�ѧʽ����b�������������� ��
��ϴ�ӳ�ȥNaCl������渽��������KCl��Ӧѡ���Լ��� ����PH��ֽ�ⶨ��Һ��PHֵ�ķ����� ��
��2�����ᴿ��NaCl����500mL��2.5mol��L-1��NaCl��Һ�������������ձ���������ƽ����������ӣ���ҩ�ף��������⣬����Ҫ �����������ƣ���Ӧ��ȡNaCl g
(3)���в����ᵼ������NaCl��ҺŨ��ƫ�ߵ���
| A��������Ϻ�����ҡ�ȣ��ٽ�����ƿ����ʵ��̨�ϣ�����Һ����ڿ̶��ߣ�����������ˮ���̶��ߡ� | |
| B��δ��ϴ���ձ��ڱڵ���Һת������ƿ�� | C������ʱ�����ӿ̶��ߡ� |
| D��ת����Һ֮ǰ������ƿ������������ˮ�� E������ʱ����ƽָ��ָ�����̡� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС���Թ�ҵ̼���(������Al2O3��Fe2O3��SiO2������)������ˮ���Ȼ��ƾ���(CaCl2��2H2O)�IJ�������Ϊ��
(1)CaCO3�����ᷴӦ�����ӷ���ʽ ��
(2)��������������Һ������Һ��pHΪ8.0��8.5����ʱA13+��Fe3+������ȫ����������Ҫ�ɷֵĻ�ѧʽΪ ����������������Һ�������Ʒ�п��ܻ��е�����Ϊ ��
(3)�ữʱ�����Ὣ��Һ��pH���ڵ�4.0���ң�����ҪĿ���� ��
(4)��������Ӧ����������Ũ������ȴ�ᾧ�۹��ˢ� �� ��ʵ�鲽�衣
(5)Ϊ�ⶨ��Ʒ��CaCl2��2H2O�ĺ�������ȡ7.350 g��Ʒ���200.0 mL��Һ����205.0 mL 0.5000 mol/LAgNO3��Һǡ����ȫ��Ӧ��
����Ʒ��CaCl2��2H2O����������Ϊ ��
������������������ƫ�����Ϊ�ڲ����������������� ����ģ�����ţ��������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ϼʯ�ң���Ҫ�ɷ�Na2O��K2O��Al2O3��SiO2����̼���ơ�̼��غ��������Ĺ����������£�
��֪��NaHCO3��Һ��pHԼΪ8~9��Na2CO3��Һ��pHԼΪ11~12��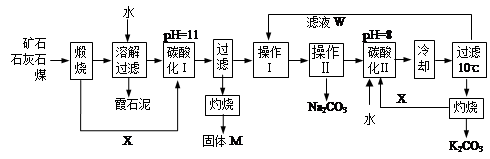
�ܽ���˹����������Һ�к��ơ��غ����Ŀ��������࣬�ƺ����������������ϼʯ���С��������ʵ��ܽ�ȼ�ͼ����������ش��������⣺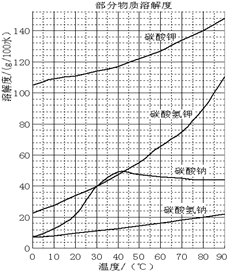
��1������M�Ļ�ѧʽ��__________�� X������___________��
��2��ʵ���ҽ������ղ���ʱʢ�Ź������ʵ�ʵ�������� __________����ҺW����Ҫ���е�������__________________��
��3��̼�ữ���з�����Ҫ��Ӧ�����ӷ���ʽ��_______________________________________��
��4����������_________����д���ƣ����������� _______(ѡ����)��
a�����ȹ��� b����ȴ���� c������ d������
��5��̼�ữ�����pH=8��Ŀ����____________________����ƷK2CO3������ܺ��е�������________(д��ѧʽ)��
��6��ʵ�������������̲ⶨ��Ʒ̼��صĴ��ȣ�Ϊ���ʵ�龫�ȣ�T�Լ������____________���������������_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ա���ۣ���Ҫ�ɷ�ΪBaCO3������Ca2+��Fe2+��Fe3+��Mg2+�ȣ��Ʊ�BaCl2��2H2O���������£� 
��1������������Ҫ��Ӧ�����ӷ���ʽΪ ��
��2������C����Ҫ�ɷ���Ca(OH)2�� ����ͼ��֪��Ϊ�˸��õ�ʹCa2+��������Ӧ��ȡ�Ĵ�ʩΪ ��

��3����BaSO4�������ⶨ��Ʒ���ȵIJ���Ϊ��
����1��ȷ��ȡ0.4��0.6 g BaCl2��2H2O����������100 ml ˮ��3 ml 2 mol��L-1��HCl��Һ�����ܽ⡣
����2���߽��裬����μ���0.1 mol��L-1 H2SO4��Һ��
����3����BaSO4������ ��ȷ������ȫ������
����4�����ˣ���0.01 mol��L-1��ϡH2SO4ϴ�ӳ���3~4�Σ�ֱ��ϴ��Һ�в���Cl��Ϊֹ��
����5�����۵��ij�����ֽ������ �У�����ɡ�̿�����һ�����800�����������ء���������BaCl2��2H2O��Ba2+�ĺ�����
�ٲ���3��ȱ�IJ���Ϊ ��
��������1��������Ʒ���٣����ڲ���4ϴ��ʱ������ɵ�Ӱ��Ϊ ��
�۲���5���ô�����������Ϊ ����ֽ�һ�ʱ����Ҫ���㣬����BaSO4�ױ�������̿��ԭ����BaS���÷�Ӧ�Ļ�ѧ����ʽΪ ��
����ͬѧ��Ϊ��K2CrO4����H2SO4��������Ч�����ã���˵��ԭ�� ��
[��֪��Ksp(BaSO4)=1.1��10-10 Ksp(BaCrO4)=1.2��10-10]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����������FeC2O4?2H2O�����������Լ�����Ӱ�������͵�ز�����������﮵���������֪��CO�����Ȼ��٣�PdCl2����Һ��Ӧ���ɺ�ɫ���ٷۡ��ش��������⣺
I����ȤС��Բ��������ķֽ�������ʵ���̽����
��1���������������ͨ��A������ʯ��ˮ��B���Ȼ��٣��۲쵽A�г���ʯ��ˮ������ǣ�B�г��ֺ�ɫ�������ɣ�����������˵������������� ��
��2��̽���ֽ�õ��Ĺ����������Ԫ�صĴ�����ʽ��
���������
����1��________�� ����2��FeO�� ����3��FeO��Fe�����
�����ʵ�鷽��֤������3��
��ѡ�Լ��� 1.0 mol?L��1���ᡢ3% H2O2��0.1 mol?L��1CuSO4��20% KSCN������ˮ��
| ʵ�鲽�� | ��������� |
| ����1 �����Թ��м��������������ټ�������_________________������� | ����Һ��ɫ���Ըı䣬����_______���ɣ���֤���������ʴ��� |
| ����2�� ������1�еõ�����Һ���ˣ���������ˮϴ����ϴ��Һ��ɫ | |
| ����3��ȡ����2�õ��������������Թ��У� �μ�___________________________________ _______________________________________ | __________________________________ ___________________________________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�������д�����CuS���������������P��������������������ʡ�ij��ѧ����С������������̣�ȡ�ÿ���Ϊԭ������CuC12��2H2O���塣
��֪�������£��������ӿ�ʼ�����ͳ�����ȫʱ��pH���±���
| �������� | �������↑ʼ������pH | �������������ȫ��pH |
| Fe2+ | 7.0 | 9.0 |
| Fe3+ | 1.9 | 3.2 |
| Cu2+ | 4.7 | 6.7 |

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com