
����Ŀ����������(CaO2)������ˮ���ڳ������ȶ����ڳ�ʪ������ˮ�л����ֽ�ų�����������㷺Ӧ������ҵ��ũҵ������������档��ͼ���Դ���ʯ(��Ҫ������������)��Ϊԭ����ȡ��������(CaO2)�����̡�

��ش��������⣺
(1)������Ӧ�������в����е�____________��(�����)
A.�ܽ� B.���� C.���� D.��Һ E.�����ᾧ
(2)�ð�ˮ����pH��8��9��Ŀ����_________________________________��
(3)�������ҺC��c(CO32-)��10��3mol/L����Ca2��____(����������������)������ȫ��[��֪c(Ca2��)��10��5 mol/Lʱ������Ϊ������ȫ��Ksp(CaCO3)��4.96��10��9]
(4)������ҺC�У�����HNO3ʹ��Һ�������Եõ�������NH4NO3�����ữ����Һ��c(NH4+) _______c(NO3-)(�����ݡ������ܡ�����<������>����������)��
(5)�������ǣ��ڵ����£�����������Ũ��Һ��Ͷ����ˮ�Ȼ��ƽ��з�Ӧ��һ��ʱ����ټ�������������Һ����������ҺpH��9��11���ų��ִ���������д���÷�Ӧ�Ļ�ѧ����ʽ_________________________���ü�Ҫ�����ֽ������������Ƶ���pH��9��11��ԭ��____________��
(6)��֪����ʯ��CaCO3����������Ϊa��m g����ʯ�����Ƶ�n g CaO2������㣺CaCO3 ת��ΪCaO2�����У�Caԭ�ӵ�������__________________��
���𰸡���1�� ABE��2�֣���2����ȥFe3����2�֣�
��3���ǣ�2�֣���4������2�֣�
��5��CaCl2��H2O2![]() CaO2��2HCl��CaCl2��H2O2��2NaOH===CaO2����2NaCl��2H2O����2�֣�������NaOH��Һʹ����ƽ��������Ӧ�����ƶ���������CaO2���������ɣ�3�֣���6��
CaO2��2HCl��CaCl2��H2O2��2NaOH===CaO2����2NaCl��2H2O����2�֣�������NaOH��Һʹ����ƽ��������Ӧ�����ƶ���������CaO2���������ɣ�3�֣���6��![]() ��2�֣�
��2�֣�
��������
���⣨1������������̼����м�������õ��Ȼ�����Һ�̶��õ��Ȼ��ƾ���Ĺ��̣����漰���IJ�����������������Һ�ܽ�̼��ƹ��壬������δ��ȫ��Ӧ��̼��ƣ��Լ�ͨ�������ᾧ�õ��Ȼ��ƾ��塣
��2������������Һ֮��ԭ��Һ�е��������ѽ�������������ʽ���ڣ�����PH��Ŀ�ľ���ͨ���γ�������������ȥ�����ӡ�
��3������KSP�Լ���Һ�е�̼������ӵ�Ũ�Ȳ�����������ӵ�Ũ��Ϊ4.96��10-6mol/L�����Կ�����Ϊ�������Ѿ�������ȫ��
��4��������Һ��������ɵĴ�����ʼ��Ϊ0��������Һ�����ԣ����Կɵó���Һ��笠�����Ũ�ȱ����������Ũ��ҪС��
��5���Ȼ�����˫��ˮ�ķ�Ӧ����ʽΪ��CaCl2��H2O2![]() CaO2��2HCl������PH�Ż���ִ���������ԭ���ǣ�������������ʹ��������Ӧ��ƽ��������Ӧ�����ƶ��Ż���ִ����Ĺ������Ƴ�����
CaO2��2HCl������PH�Ż���ִ���������ԭ���ǣ�������������ʹ��������Ӧ��ƽ��������Ӧ�����ƶ��Ż���ִ����Ĺ������Ƴ�����
��6����֪�������Ƶ���Է�������Ϊ72����̼��Ƶ����ԭ������Ϊ100�����Ը�ԭ�ӵ�������Ϊ��n/72��/��ma/100�����Կ�����ø�ԭ�ӵ�ԭ��������Ϊ![]()



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.�о����������������ڴ����к������ӵ������ʱ��ͬ�¶����漰���·�Ӧ��
��2NO(g)��Cl2(g)2ClNO(g) ƽ�ⳣ��ΪK1��
��2NO2(g)��NaCl(s)NaNO3(s)��ClNO(g) ƽ�ⳣ��ΪK2��
(1)4NO2(g)��2NaCl(s)2NaNO3(s)��2NO(g)��Cl2(g)��ƽ�ⳣ��K��____(��K1��K2��ʾ)��
(2)����Ӧ�����¶�T�´ﵽƽ��ʱ��ƽ�ⳣ��ΪK1�������¶Ⱥ�K1����������Ӧ���ʱ��H____0������>������<������=��������ƽ����������������������£���������Cl2��Cl2��ת����___________�����������������С������������,��ͬ����![]() ��ֵ___________��
��ֵ___________��
��.���꣬��ѧ���о����Ҵ����ϳ������������·�����2C2H5OH(g)![]() CH3COOC2H5(g)��2H2(g)�ڳ�ѹ�·�Ӧ�������ռ�����ó�����Һ̬�ռ�������Ҫ���������������ͼ3��ʾ�����ڸ÷����������Ʋ��������________��
CH3COOC2H5(g)��2H2(g)�ڳ�ѹ�·�Ӧ�������ռ�����ó�����Һ̬�ռ�������Ҫ���������������ͼ3��ʾ�����ڸ÷����������Ʋ��������________��

A����Ӧ�¶Ȳ��˳���300��
B��������ϵѹǿ������������Ҵ�ƽ��ת����
C���ڴ��������£���ȩ�Ƿ�Ӧ�����е��м����
D����ߴ����Ļ��Ժ�ѡ���ԣ��������ѡ���ϩ�ȸ������ǹ��յĹؼ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��L��M����Ԫ�ص�ԭ��������������X��Y��Z��L����ɵ����ʵĻ���Ԫ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�ء�
�ش��������⣺
(1)L������Ϊ__________________________������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳��Ϊ______________________________(��Ԫ�ط��ű�ʾ)��
(2)Z��X��Ԫ�ذ�ԭ����Ŀ��1��3��2��4���ɷ���A��B����ҵ�ϳ�A�Ļ�ѧ����ʽΪ______________________________��B�ĽṹʽΪ_____________________���ڱ�״���£���A����һ��������ƿ������ƿ������ˮ�У�ƿ��Һ��������(�������ʲ���ɢ)�����ƿ����Һ�����ʵ����ʵ���Ũ��Ϊ________(��ȷ��0.001)��
(3)��(Se)������������Ԫ�أ���Lͬһ���壬Seԭ�ӱ�Lԭ�Ӷ��������Ӳ㣬��������������Ӧˮ����Ļ�ѧʽΪ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1���������˻ᡰ��������������ܴ����Դ�ڱ����ȼ�գ�������һ��������ȼ�ϡ��Իش��������⣺
����ͼ��һ����������ȫȼ������CO2��1 mol H2O(l)�����е������仯ͼ������ͼ�е�������������������������_____��

��д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��___________________________________��
��������(CH3OCH3)��һ������ȼ�ϣ�Ӧ��ǰ��������1 mol��������ȫȼ������CO2��Һ̬ˮ�ų�1 455 kJ��������1 mol����Ͷ����ѵĻ��������ȫȼ������CO2��Һ̬ˮ���ų�1835 kJ���������������У�����Ͷ����ѵ����ʵ���֮��Ϊ_______��
��2����ѧ�Ҹ�˹������������ܻ�ѧ������һ����ɻ�ּ�����ɣ�����ܹ��̵���ЧӦ����ͬ�ġ������ø�˹���ɿɲ�ijЩ�ر�Ӧ����ЧӦ��
��P4(s������)��5O2(g)===P4O10(s)����H1����2 983.2 kJ��mol��1
��P(s������)��5/4O2(g)===1/4P4O10(s)����H2����738.5 kJ��mol��1
�����ת��Ϊ�����Ȼ�ѧ����ʽΪ_________________________________________����ͬ��״���£������ϵ͵���________�������ȶ��ԱȺ���________(����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��300��ʱ,��2mol A��2mol B���������ϼ���2L�ܱ�������,������Ӧ3A(g)+B(g)![]() 2C(g)+2D(g)��H,2minĩ��Ӧ�ﵽƽ��,����0.8mol D����������ʽ��𡣣�
2C(g)+2D(g)��H,2minĩ��Ӧ�ﵽƽ��,����0.8mol D����������ʽ��𡣣�
(1)��÷�Ӧ��ƽ�ⳣ��____��
(2)��ƽ��ʱA��ת����____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ�У���д��ȷ����( )��
A.����ϡ���ᷴӦ��2Fe��6H����2Fe3����3H2��
B.ϡ����������������Һ��Ӧ��Ba2����H����OH-��![]() ��H2O��BaSO4��
��H2O��BaSO4��
C.̼�����ϡ���ᷴӦ��CaCO3��2H����Ca2����CO2����H2O
D.ͭƬ����������Һ��Ӧ��Cu��Ag����Cu2����Ag
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£����и���������ָ����Һ���ܴ���������ǣ� ��
A. ��ɫ������Һ�У�Fe3+��Mg2+��SCN-��Cl-
B. c(Fe2+)=1mol��L-1����Һ�У�K+��NH4+��MnO4-��SO42-
C. c(H+)/c(OH-)=1��10-12����Һ�У�K+��Na+��CO32-��NO3-
D. ��ʹ���ȱ�����Һ�У�Na+��NH4+��SO42-��HCO3-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Zn���ִ���ҵ�ж��ڵ���������в���ĥ��ĵ�λ������ĩ����Ӧ�������ġ��칤���һ���о�������������Ĺ�����п�����ļ��ء��ش��������⣺
(1)Zn��̬ԭ�ӵĵ����Ų�ʽΪ___________��4s�ܼ��ϵijɶԵ�����Ϊ___________��
(2)��������п{[CH2OH( CHOH)4COO]2Zn}��Ŀǰ�г������еIJ�п������������п��̼ԭ���ӻ���ʽ��___________��C��H��Ԫ�صĵ�һ�����ܵĴ�С��ϵΪ___________________��
(3)ZnCl2��NH3�γɵ������[Zn(NH3)4]Cl2�У�����___________(����ĸ)��
A���Ӽ� B.���� C.����
(4)п��ij�ǽ���Ԫ��X�γɵĻ����ᄃ����ͼ��ʾ������Zn��Xͨ�����ۼ���ϣ��û�������Zn��X��ԭ�Ӹ���֮��Ϊ___________��
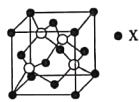
(5)��ͼʾ��������ֻ����X�����з�ʽ����X�Ķѻ���ʽ���ڽ�������ѻ���ʽ�е�___________�ѻ����谢���ӵ���������ֵΪNA���þ�����Zn�İ뾶Ϊr1nm��X�İ뾶Ϊr2nm��X�����ԭ������ΪM����þ�����ܶ�Ϊ___________g��cm��3(�ú�r1��r2��M��NA�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ñ�NaOH��Һ�ζ�����HCl����ƿ(�����)������Ȫʵ���õ���ϡ���ᣬ�Բⶨ����ȷŨ�ȣ�����ش��������⣺
(1)���ۼ������������ʵ���Ũ��Ϊ��________________________________��
(2)���ü�����ָʾ�����ﵽ�����յ�ʱ��������___________________________��
(3)��������Ũ�ȵı�NaOH��Һ������Ϊ����ʵ������е�__________�֡�
��5.00mol��L-1 ��0.500mol��L-1 ��0.0500mol��L-1
(4)���������������Ũ�ȵı�NaOH��Һ�������ζ�ʱʵ�������б����£�
ʵ�������� | �����������(mL) | ����NaOH��Һ���(mL) |
1 | 10.00 | 8.48 |
2 | 10.00 | 8.52 |
3 | 10.00 | 8.00 |
�����ִ���ϡ��������ʵ���Ũ��c(HCl)=___________________��
(5)�ڵζ����������У����¸������ʹ�ⶨֵƫ�ߵ��У�_______________
�ٵζ���������ˮϴ����δ����֪Ũ�ȵı���Һ��ϴ
�ڵζ���(װ����Һ)�ڵζ�ǰ���촦�����ݣ��ζ�����������
�۵ζ�ǰƽ�ӣ��ζ����˸���
�ܿ�����ɫ�仯����������
��ϴ����ƿʱ�����ϡʳ��ˮ��������ˮ����ϴ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com