
����Ŀ�����������л���ش�������⣬
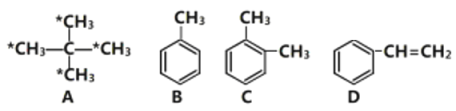
��1���л���A������___________����ע��*����̼ԭ�������������ɵ�ͼ��Ϊ________������Ρ����������Ρ������������Ρ�����E��A��ͬϵ��ұ�A��һ��̼ԭ�ӣ���E��һ�ȴ�����______�֡�
��2����ͬ���������������л�����ȫȼ��ʱ������������_______________(��ṹ��ʽ����
��3���л���B��ʵ������ת����
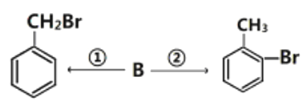
����ת���ķ�Ӧ����Ϊ��_______________����_______________��
��4���л���D�Ĺ���������Ϊ_______________��D��һ�������������ɸ߷��ӻ�����ķ�Ӧ��ѧ����ʽΪ___________________________________________________________��
��5����ͼ�����Ҵ��йص�����ʵ�飬
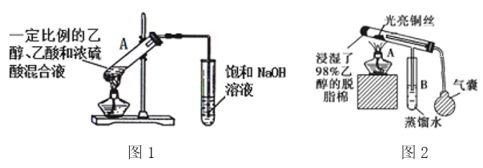
��ͼ1�Թ�A�еķ�Ӧ����ʽΪ______________________________��Ũ�����������_________����ָ��ͼ1���������ԵĴ���_________________________________________��
�ڵ�ȼͼ2�оƾ��ƣ�������ѹ���ң���ͼ2װ���й��������ͭ˿���������ɺ������ɺڱ�����������ͭ˿�ɺڱ���ԭ����__________________________________���û�ѧ����ʽ��ʾ����
���𰸡������飨��2,2���������飩����������4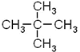 ������������Һ�壨���壩��FeBr3��Fe̼̼˫��
������������Һ�壨���壩��FeBr3��Fe̼̼˫��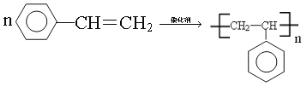 CH3COOH+HOCH2CH3
CH3COOH+HOCH2CH3![]() CH3COOCH2CH3+H2O��������ˮ������ĩ������Һ�����£�����ɵ���������NaOH��Һ����̫ǿ���������������ˮ������Ͳ���CH3CH2OH+CuO
CH3COOCH2CH3+H2O��������ˮ������ĩ������Һ�����£�����ɵ���������NaOH��Һ����̫ǿ���������������ˮ������Ͳ���CH3CH2OH+CuO![]() CH3CHO+Cu+H2O
CH3CHO+Cu+H2O
��������
(1)�л�A�൱�ڼ�������е��ĸ���ԭ�ӱ��ĸ�����ȡ�����ʱ�ע��*����̼ԭ�������������ɵ�ͼ����Ϊ�����壻EΪ���飬�������ֽṹ��CH3(CH2)3-��CH3CH2CH(CH3)-��(CH3)3C-��(CH3)2CHCH2-������һ�ȴ���Ҳ�����֣��ʴ�Ϊ�������飨��2,2���������飩��������������4��(2) ��ͬ����������ȫȼ��ʱ���������˵����������Ԫ�ص�����������ߣ��������л���ķ���ʽ�ֱ�Ϊ��C5H12��C7H8��C8H10��C8H8��ͨ������ʽ�ĶԱȿ�֪A����Ԫ�ص�����������ߣ���Ϊ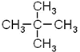 ��(3)����ԭ��ȡ����λ�ÿɵ÷�Ӧ�����ֱ�Ϊ����������������Һ�壨���壩��FeBr3��Fe��(4)�����������ڹ����ţ��ʴ�Ϊ��̼̼˫����
��(3)����ԭ��ȡ����λ�ÿɵ÷�Ӧ�����ֱ�Ϊ����������������Һ�壨���壩��FeBr3��Fe��(4)�����������ڹ����ţ��ʴ�Ϊ��̼̼˫����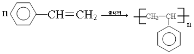 ��(5)ͼ1Ϊ�Ҵ���������Ӧʵ�飬ͼ2Ϊ�Ҵ��Ĵ�����ʵ�飬�ʴ�Ϊ��CH3COOH+HOCH2CH3
��(5)ͼ1Ϊ�Ҵ���������Ӧʵ�飬ͼ2Ϊ�Ҵ��Ĵ�����ʵ�飬�ʴ�Ϊ��CH3COOH+HOCH2CH3![]() CH3COOCH2CH3+H2O����������ˮ��������ĩ������Һ�����£�����ɵ���������NaOH��Һ����̫ǿ���������������ˮ������Ͳ�����CH3CH2OH+CuO
CH3COOCH2CH3+H2O����������ˮ��������ĩ������Һ�����£�����ɵ���������NaOH��Һ����̫ǿ���������������ˮ������Ͳ�����CH3CH2OH+CuO![]() CH3CHO+Cu+H2O��
CH3CHO+Cu+H2O��


 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
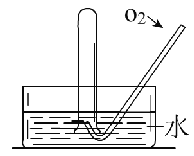
����Ŀ����ͼ��ʾ����һƿ����NO2������Թܵ�����ˮ���У�������ɫ��____��Ϊ____ɫ���Թ��ڵ�Һ�����_____________������Ӧֹͣ�����Թ��л���ͨ��������������ɫ����______ɫ��Ϊ______ɫ��֮���ֱ�Ϊ______ɫ���Թ��ڵ�Һ���____________���û�ѧ����ʽ��ʾ�Թ��з����Ļ�ѧ��Ӧ_____________��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪ�Ӻ�������ȡ���Ĺ�ҵ�������̣��й�˵����������

A����ˮ���̲��ż���ḻ�ĵ�Ԫ�أ����Թ�ҵ��Ҳ����ֱ���ú�ˮ����ȡ��Ԫ��
B�������ʱ������NaOH��Һ��ԭ�����ڼ�����Һ�У��������л��ʿ��γɳ���
C����ʵ���ҽ�����������ʱ�������������������������ˮ��˫��ˮ��
D����ʵ���ҽ��й��˲���ʱ����Ҫ�õ����������в��������ձ���©��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵķ��뷽������ȷ���ǣ� ��
A.������ķ���������ˮ�Ƴ�����ˮ
B.�ù��˵ķ�����ȥʳ��ˮ�е���ɳ
C.����ϴ�ķ�����ɳ���Խ�
D.�þƾ���ȡ��ˮ�еĵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������йص������Һ������Ũ�ȹ�ϵ��ȷ����(����)
A. pH��1��NaHSO4��Һ�� c(H��)��c(SO![]() )��c(OH��)
)��c(OH��)
B. ����AgCl��AgI���������Һ�� c(Ag��)>c(Cl��)��c(I��)
C. CO2��ˮ��Һ��c(H��)>c(HCO![]() )��2c(CO
)��2c(CO![]() )
)
D. �������ʵ�����NaHC2O4��Na2C2O4����Һ��3c(Na��)��2[c(HC2O![]() )��c(C2O
)��c(C2O![]() )��c(H2C2O4)]
)��c(H2C2O4)]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ��һ����Ļ�ѧ��Դ���⣬�����йغ�ˮ�ۺ����õ�˵����ȷ���ǣ� ��
A.�ú�ˮΪԭ�ϣ���һϵ�й����Ƶ�����þ���壬H2��ԭ��þ
B.�Ӻ�ˮ�п��Եõ�NaCl���������NaCl���Ʊ�Na
C.��ˮ�����ƺ��εĹ�����ֻ�����˻�ѧ�仯
D.Ŀǰ��ҵ��ֱ���ɺ�ˮ��ȡI2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ȡƯ�۵ķ�ӦΪ_______��Ư�۵���Ҫ�ɷ�Ϊ_______����Ч�ɷ�Ϊ_______��Ư��ԭ��Ϊ_______��HClO������Ư�����ã������������ã�HClO�IJ��ȶ���_______���÷���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������е������dz�ȥ���������ҩƷ����������д������
A.NaCl��Һ��������Na2SO4���Ȼ�����B.FeSO4��Һ��������CuSO4�����ۣ�
C.��ʯ���л�������ʯ��ʯ���������գ�D.CO2��������CO�����ȵ�����ͭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���A�ǾۺϷ�Ӧ�����������ϵĵ��壬�����Ϊ�ϳɵ����I����������J��ԭ�ϣ���غϳ�·�����£�

��֪��������ͼ����A������ʺɱ�Ϊ118���䱽���ϵ�һ�ȴ��ﹲ���֣��˴Ź���������
ʾ�������Ϊ3:2:2:2:1��
����������Ϣ�ش��������⣺
��1��A�Ĺ���������Ϊ__________________��B��C�ķ�Ӧ����Ϊ_____________��E��F�ķ�Ӧ����Ϊ_____________��
��2��I�Ľṹ��ʽΪ____________________����K�����к���������Ԫ��״�ṹ���������ʽΪ________________��
��3��D������������ͭ����Һ��Ӧ�����ӷ���ʽΪ_______________________________��
��4��H��ͬ���칹��W����Ũ��ˮ��Ӧ������ɫ������1 mol W���뷴Ӧ�������3 mol Br2����д�����з���������W�Ľṹ��ʽ___________________________________��
��5��J��һ�ָ߷��ӻ��������C����J�Ļ�ѧ����ʽΪ
______________________________________________________________________��
��6��![]() ��֪��
��֪��![]() ��RΪ������
��R������
����Ա�����ϩΪ��ʼԭ���Ʊ�H�ĺϳ�·�ߣ����Լ���ѡ����
[�ϳ�·��ʾ����]![]()
_____________________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com