
ЁОЬтФПЁПГЃЮТЯТЃЌНЋФГвЛдЊЫсHAКЭNaOHШмвКЕШЬхЛ§ЛьКЯЃЌЪЕбщаХЯЂШчЯТЃК
ЪЕбщБрКХ | c(HA)/ molЁЄLЃ1 | c(NaOH)/ molЁЄLЃ1 | ЗДгІКѓШмвКpH |
Мз | 0.1 | 0.1 | pHЃН9 |
вв | c1 | 0.2 | pHЃН7 |
ЯТСаХаЖЯВЛе§ШЗЕФЪЧ
A.c1вЛЖЈДѓгк0.2 molЁЄLЃ1
B.HAЕФЕчРыЗНГЬЪНЪЧHA![]() HЃЋЃЋAЃ
HЃЋЃЋAЃ
C.МзЗДгІКѓШмвКжаЃКc(NaЃЋ) ЃО c(OHЃ)ЃО c(AЃ) ЃО c(HЃЋ)
D.ввЗДгІКѓШмвКжаЃКc(NaЃЋ) ЃМ c(HA)ЃЋc(AЃ)
ЁОД№АИЁПC
ЁОНтЮіЁП
гЩЪЕбщМзПЩжЊЃЌЫсКЭМюЧЁКУЗДгІЃЌЗДгІКѓЩњГЩpHЃН9ЕФNaAШмвКЃЌЫЕУїA-РызгЫЎНтЪЙШмвКЯдМюадЃЌЙЪHAЮЊШѕЫсЁЃ
AЁЂШмвКжавЛЖЈДцдкcЃЈOH-ЃЉ+cЃЈA-ЃЉ=cЃЈNa+ЃЉ+cЃЈH+ЃЉЃЌЛьКЯШмвКpH=7ЫЕУїШмвКГЪжаадЃЌcЃЈOH-ЃЉ=cЃЈH+ЃЉЃЌЫљвдcЃЈA-ЃЉ=cЃЈNa+ЃЉЃЌЗДгІКѓЩњГЩNaAШмвКЃЌA-РызгЫЎНтЪЙШмвКЯдМюадЃЌЙЪHAЙ§СПВХФмЪЙШмвКЯджаадЃЌвђДЫЃЌдШмвКХЈЖШc1ЃО0.2 molL-1ЃЌAе§ШЗЃЛ
BЁЂHAЪЧШѕЫсДцдкЕчРыЦНКтЃЌHAЕФЕчРыЗНГЬЪНЪЧHAH++A-ЃЌBе§ШЗЃЛ
CЁЂЗДгІКѓЩњГЩNaAШмвКЃЌA-РызгЫЎНтЯдМюадЃЌШмвКжаРызгХЈЖШДѓаЁcЃЈNa+ЃЉЃОcЃЈA-ЃЉЃОcЃЈOH-ЃЉЃОcЃЈH+ЃЉЃЌCДэЮѓЃЛ
DЁЂввШмвКжавЛЖЈДцдкcЃЈOH-ЃЉ+cЃЈA-ЃЉ=cЃЈNa+ЃЉ+cЃЈH+ЃЉЃЌЛьКЯШмвКpH=7ЫЕУїШмвКГЪжаадЃЌcЃЈOH-ЃЉ=cЃЈH+ЃЉЃЌЫљвдcЃЈA-ЃЉ=cЃЈNa+ЃЉЃЌcЃЈNa+ЃЉЃМcЃЈHAЃЉ+cЃЈA-ЃЉЃЌDе§ШЗЁЃ
ЙЪбЁCЁЃ


 вЛПЮвЛСЗПЮЪБДяБъЯЕСаД№АИ
вЛПЮвЛСЗПЮЪБДяБъЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгУ1.0mol/LЕФNaOHШмвКжаКЭФГХЈЖШЕФH2SO4ШмвКЃЌЦфЫЎШмвКЕФpHКЭЫљгУNaOHШмвКЕФЬхЛ§БфЛЏЙиЯЕШчЭМЫљЪОЃЌдђдH2SO4ШмвКЕФЮяжЪЕФСПХЈЖШКЭЭъШЋЗДгІКѓШмвКЕФДѓжТЬхЛ§ЪЧЃЈ ЃЉ
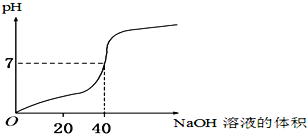
AЃЎ1.0 mol/LЃЌ20 mL BЃЎ0.5 mol/LЃЌ40 mL
CЃЎ0.5 mol/LЃЌ80 mL DЃЎ1.0 mol/LЃЌ80 mL
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПДгЮяжЪA(ФГе§бЮ)ЕФЫЎШмвКГіЗЂгаЯТУцЫљЪОЕФвЛЯЕСаБфЛЏЃК
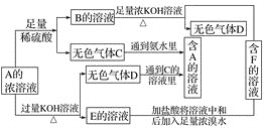
ЃЈ1ЃЉаДГіAЁЋFЮяжЪЕФЛЏбЇЪНЃК
A__________ЃЛB__________ЃЛC__________ЃЛD__________ЃЛE.__________ЃЛF__________ЁЃ
ЃЈ2ЃЉаДГіEЁњFЕФЛЏбЇЗНГЬЪН______________________________ЁЃ
ЃЈ3ЃЉМјБ№ЮяжЪFжавѕРызгЕФЗНЗЈЪЧ________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПГЃЮТЯТгУNaOHШмвКЕЮЖЈH2C2O4ШмвКЕФЙ§ГЬжаЃЌШмвКжаЃlg![]() КЭЃlg c(HC2O4-)ЛђЃlg
КЭЃlg c(HC2O4-)ЛђЃlg![]() КЭЃlg c(C2O42-)ЕФЙиЯЕШчЭМЫљЪОЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈЁЁЁЁЃЉ
КЭЃlg c(C2O42-)ЕФЙиЯЕШчЭМЫљЪОЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈЁЁЁЁЃЉ
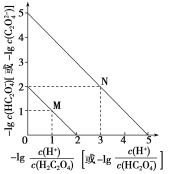
A.Ka1(H2C2O4)Ъ§СПМЖЮЊ10Ѓ1
B.ЧњЯпNБэЪОЃlg![]() КЭЃlg c(HC2O4-)ЕФЙиЯЕ
КЭЃlg c(HC2O4-)ЕФЙиЯЕ
C.ЯђNaHC2O4ШмвКжаМгNaOHжСc(HC2O4-)КЭc(C2O4-)ЯрЕШЃЌДЫЪБШмвКpHдМЮЊ5
D.дкNaHC2O4ШмвКжаc(NaЃЋ)ЃОc(HC2O4-)ЃОc(H2C2O4)ЃОc(C2O42-)
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЧњУРЬцФсЪЧвЛжжвжжЦКкЩЋЫиСіЕФаТаЭПЙАЉвЉЮяЃЌЯТУцЪЧКЯГЩЧњУРЬцФсжаМфЬхGЕФЗДгІТЗЯпЃК
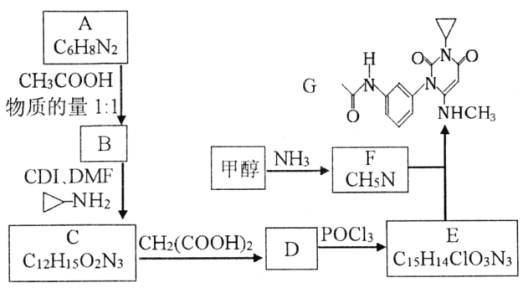
вбжЊЃКЂйDЗжзгжага2Иі6дЊЛЗЃЛ
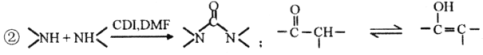
ЧыЛиД№ЃК
ЃЈ1ЃЉЛЏКЯЮяAЕФНсЙЙМђЪН___________ЁЃAЩњГЩBЕФЗДгІРраЭ___________ЁЃ
ЃЈ2ЃЉЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ___________ЁЃ
A.BМШФмБэЯжМюадгжФмБэЯжЫсад
B.1moCдкМюШмвКжаЭъШЋЫЎНтзюЖрПЩвдЯћКФ4 molOHЃ
C.DгыPOCl3ЕФЗДгІЛЙЛсЩњГЩEЕФвЛжжЭЌЗжвьЙЙЬх
D.GЕФЗжзгЪНЮЊC16H18O3N4
ЃЈ3ЃЉаДГіCЁњDЕФЛЏбЇЗНГЬЪН____________________________________________ЁЃ
ЃЈ4ЃЉXЪЧБШAЖр2ИіЬМдзгЕФAЕФЭЌЯЕЮяЃЌаДГіЗћКЯЯТСаЬѕМўЕФXПЩФмЕФНсЙЙМђЪНЃК_______________________________________________________ЁЃ
Ђй1H-NMRЦзЯдЪОЗжзгжага3жжЧтдзгЃЌЂкIRЦзЯдЪОЗжзгжагаБНЛЗгыЃNH2ЯрСЌНсЙЙ
ЃЈ5ЃЉСїГЬжаЪЙгУЕФDMFМДNЃЌN-ЖўМзЛљМзѕЃАЗНсЙЙМђЪНЮЊ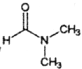 ЃЌЪЧГЃгУЕФгаЛњШмМСЁЃЩшМЦвдМзДМКЭАБЮЊжївЊдСЯжЦШЁDMFЕФКЯГЩТЗЯп(гУСїГЬЭМБэЪОЃЌЦфЫћЮоЛњЪдМСШЮбЁ)ЁЃ_____________
ЃЌЪЧГЃгУЕФгаЛњШмМСЁЃЩшМЦвдМзДМКЭАБЮЊжївЊдСЯжЦШЁDMFЕФКЯГЩТЗЯп(гУСїГЬЭМБэЪОЃЌЦфЫћЮоЛњЪдМСШЮбЁ)ЁЃ_____________
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЧІЫсаюЕчГиЕФЙЄзїдРэШчЭМЫљЪОЃЌЦфзмЗДгІЪНЮЊ![]() ЁЃЯТСаХаЖЯВЛе§ШЗЕФЪЧ
ЁЃЯТСаХаЖЯВЛе§ШЗЕФЪЧ

A.БеКЯKЪБЃЌdЕчМЋЕФЗДгІЪНЮЊ![]()
B.ЕБЕчТЗжазЊвЦ![]() ЕчзгЪБЃЌЂёжаЯћКФЕФ
ЕчзгЪБЃЌЂёжаЯћКФЕФ![]() ЮЊ
ЮЊ![]()
C.БеКЯKЪБЃЌЂђжа![]() ЯђcЕчМЋЧЈвЦ
ЯђcЕчМЋЧЈвЦ
D.БеКЯKвЛЖЮЪБМфКѓЃЌЂђПЩЕЅЖРзїЮЊдЕчГиЃЌdЕчМЋЮЊе§МЋ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгУЛюадЬПЛЙдДІРэЕЊбѕЛЏЮяЃЌгаЙиЗДгІЮЊC(s)+2NO(g) ![]() N2(g)+CO2(g)ЁЃ
N2(g)+CO2(g)ЁЃ
ЃЈ1ЃЉаДГіЩЯЪіЗДгІЕФЦНКтГЃЪ§БэДяЪН_______________ЁЃ
ЃЈ2ЃЉдк2LКуШнУмБеЦїжаМгШызуСПCгыNOЗЂЩњЗДгІЃЌЫљЕУЪ§ОнШчБэЃЌЛиД№ЯТСаЮЪЬтЁЃ
ЪЕбщБрКХ | ЮТЖШ/Ёц | Ц№ЪМЪБNOЕФЮяжЪЕФСП/mol | ЦНКтЪБN2ЕФЮяжЪЕФСП/mol |
1 | 700 | 0.40 | 0.09 |
2 | 800 | 0.24 | 0.08 |
ЂйНсКЯБэжаЪ§ОнЃЌХаЖЯИУЗДгІЕФЁїH____0(ЬюЁАЃОЁБЛђЁАЃМЁБ)ЃЌРэгЩЪЧ_________ЁЃ
ЂкХаЖЯИУЗДгІДяЕНЦНКтЕФвРОнЪЧ_______ЁЃ
A.ШнЦїФкЦјЬхУмЖШКуЖЈ B.ШнЦїФкИїЦјЬхХЈЖШКуЖЈ
C.ШнЦїФкбЙЧПКуЖЈ D.2vе§ЃЈNOЃЉ= vФцЃЈN2ЃЉ
ЃЈ3ЃЉ700ЁцЪБЃЌШєЯђ2LЬхЛ§КуЖЈЕФУмБеШнЦїжаГфШывЛЖЈСПN2КЭCO2ЗЂЩњЗДгІЃКN2(g)+CO2(g)![]() C(s)+2NO(g) ЃЛЦфжаN2ЁЂNOЮяжЪЕФСПЫцЪБМфБфЛЏЕФЧњЯпШчЯТЭМЫљЪОЁЃЧыЛиД№ЯТСаЮЪЬтЁЃ
C(s)+2NO(g) ЃЛЦфжаN2ЁЂNOЮяжЪЕФСПЫцЪБМфБфЛЏЕФЧњЯпШчЯТЭМЫљЪОЁЃЧыЛиД№ЯТСаЮЪЬтЁЃ
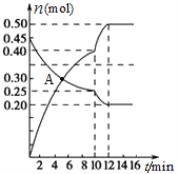
Ђй0ЁЋ10 minФкЕФCO2ЦНОљЗДгІЫйТЪvЃН____________ЁЃ
ЂкЭМжаAЕуv(е§)___v(Фц)ЃЈЬюЁАЃОЁБЁЂЁАЃМЁБЛђЁАЃНЁБЃЉЁЃ
ЂлЕк10 minЪБЃЌЭтНчИФБфЕФЬѕМўПЩФмЪЧ_____________ЁЃ
AЃЎМгДпЛЏМС BЃЎдіДѓCЕФЮяжЪЕФСП
CЃЎМѕаЁCO2ЕФЮяжЪЕФСП DЃЎЩ§ЮТ EЃЎНЕЮТ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПУЬЫсяЎЃЈLiMn2O4)ЪЧаТаЭяЎРызгЕчГиГЃгУЕФе§МЋВФСЯЁЃЙЄвЕЩЯвдШэУЬПѓНЌЮЊдСЯПЩжЦБИУЬЫсяЎЃЌЭЌЪБжЦЕУИБВњЦЗMnSO4ЁЄH2OОЇЬхЃЌЦфСїГЬШчЭМЫљЪОЁЃ
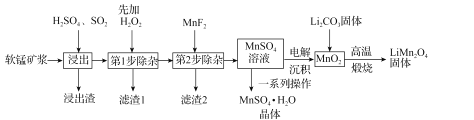
вбжЊЃКЃЈ1ЃЉШэУЬПѓНЌЕФжївЊГЩЗжЮЊMnO2ЃЌЛЙКЌгаFe2O3ЃЌMgOЁЂAl2O3ЃЌCaOЃЌSiO2ЕШдгжЪЁЃ
ЃЈ2ЃЉЮТЖШИпгк27ЁцЪБЃЌMnSO4ОЇЬхЕФШмНтЖШЫцЮТЖШЩ§ИпЖјж№НЅНЕЕЭЁЃ
ЃЈ3ЃЉгаЙиЮяжЪЕФШмЖШЛ§ГЃЪ§ШчЯТБэЃК
ЃЈ1ЃЉЁАНўГіЁБЙ§ГЬжаMnO2зЊЛЏЮЊMn2+ЕФРызгЗНГЬЪНЮЊ_____ЁЃИУЙ§ГЬжаЃЌЮЊЬсИпШэУЬПѓжаMnO2ЕФНўГіТЪЃЌЯТСаДыЪЉПЩааЕФга_____ЃЈЬюзжФИЃЉЁЃ
AЃЎВЛЖЯНСАшЃЌЪЙSO2КЭШэУЬПѓНЌГфЗжНгДЅ
BЃЎдіДѓЭЈШыSO2ЕФСїЫй
CЃЎЪЪЕБЩ§ЮТ
DЃЎМѕЩйШэУЬПѓНЌЕФНјШыСП
ЃЈ2ЃЉЕк1ВНГ§дгжаМгШыH2O2ЕФФПЕФЪЧ_____ЁЃ
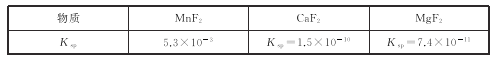
ЃЈ3ЃЉЕк2ВНГ§дгЃЌжївЊЪЧНЋCa2+ЃЌMg2+зЊЛЏЮЊЯргІЕФЗњЛЏЮяГСЕэГ§ШЅЃЌЦфжаMnF2Г§ШЅMg2+ЗДгІЕФРызгЗНГЬЪНЮЊMnF2(s)+Mg2+(aq)=Mn2+(aq)+MgF2(s)ЃЌИУЗДгІЕФЦНКтГЃЪ§ЮЊ_____ЁЃ
ЃЈ4ЃЉЭМжаЕФвЛЯЕСаВйзїжИЕФЪЧ_____ЁЃ
ЃЈ5ЃЉНЋMnO2КЭLi2CO3АД4ЃК1ЕФЮяжЪЕФСПжЎБШХфСЯЃЌЛьКЯНСАшЃЌШЛКѓИпЮТьбЩе600~750ЁцЃЌжЦШЁВњЦЗLiMn2O4ЁЃаДГіИУЗДгІЕФЛЏбЇЗНГЬЪНЃК_____ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПТШЛЏяЇЫзГЦТБЩАЃЌжївЊгУгкИЩЕчГиЁЂЛЏЗЪЕШЁЃФГЛЏбЇбаОПаЁзщЩшМЦШчЭМЪЕбщжЦБИТБЩАВЂНјаадЊЫиВтЖЈЁЃ
Ђё.ЪЕбщЪвжЦБИТБЩАЫљашЕФзАжУШчЭМЫљЪОЃЌзАжУПЩжиИДбЁгУЁЃ

ЃЈ1ЃЉзАжУНгПкСЌНгЫГађЪЧ___ЁњaЃЛbЁћ___ЁЃ
ЃЈ2ЃЉCзАжУЕФзїгУЪЧ___ЃЌDзАжУЪЂзАЕФЮяжЪЪЧ___ЁЃ
ЃЈ3ЃЉаДГігУЩЯЪізАжУжЦБИАБЦјЕФвЛзщЪдМСЃК___ЁЃ
Ђђ.ВтЖЈТБЩАжаClдЊЫиКЭNдЊЫиЕФжЪСПжЎБШЁЃ
ИУбаОПаЁзщзМШЗГЦШЁagТБЩАЃЌгызуСПбѕЛЏЭЛьКЯМгШШЃЌГфЗжЗДгІКѓАбЦјЬхВњЮяАДШчЭМзАжУНјааЪЕбщЁЃЪеМЏзАжУЪеМЏЕНЕФЦјЬхЮЊПеЦјжаКЌСПзюЖрЕФЦјЬхЃЌЦфЬхЛ§ЛЛЫуГЩБъзМзДПіЯТЕФЬхЛ§ЮЊVLЃЌМюЪЏЛвдіжиbgЁЃ

ЃЈ4ЃЉEзАжУФкЕФЪдМСЮЊ___ЃЌТБЩАгыбѕЛЏЭЛьКЯМгШШЗДгІЕФЛЏбЇЗНГЬЪНЮЊ___ЁЃ
ЃЈ5ЃЉТБЩАжаClдЊЫиКЭNдЊЫиЕФжЪСПжЎБШЮЊ___(гУКЌbЁЂVЕФЪНзгБэЪО)ЁЃ
ЃЈ6ЃЉЮЊСЫВтЖЈТБЩАжаТШдЊЫиЕФжЪСПЃЌЫћУЧЩшМЦЕФЪЕбщЗНАИЪЧНЋagТБЩАЭъШЋШмНтгкЫЎЃЌМгШыЙ§СПAgNO3ШмвКЃЌШЛКѓВтЖЈЩњГЩГСЕэЕФжЪСПЁЃЧыФуЦРМлИУЗНАИЪЧЗёКЯРэЃЌВЂЫЕУїРэгЩЃК___ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com