

���� ��1��������ת����ϵ������֪���ƻƣ�As2S3��������Һ�к�Sn2+���ӷ���������ԭ��Ӧ����As4S4��H2S��Sn4+����ϵ���غ㡢ԭ���غ���ƽ��д���ӷ���ʽ��
����1molAs4S4��Ӧת��28mole-����Ӧ����7mol����������ԭ���غ���д���ӷ���ʽ����ϵ����غ���㣻
��2������Na2HPO4��Һ�м�������CaCl2��Һ��������Һ�����ԣ�˵����Ӧ��������Ƴ������Ȼ��ƺ��Ȼ��⣻
��pH=7����������Һ����Ϊ������Һ������Һ����Ԫ�صĴ�����̬����ͼ��仯�����õ���ͼ���п�֪H2PO4-��HPO42-���ʵ�����ͬ�����Ԫ���غ����õ�����������������ʵ���֮�ȣ�
��pH��2��4֮�䣬�����δ�������ˮʱ������ҺpH����H2PO4-�����������Ч����ǿ��
��3����CuI��s��+2NH3•H2O��aq��?Cu��NH3��2+��aq��+I-��aq��+2H2O��1����ƽ�ⳣ��K=$\frac{c��{I}^{-}��c��Cu��N{H}_{3}{��_{2}}^{+}��}{{c}^{2}��N{H}_{3}•{H}_{2}O��}$=$\frac{c��{I}^{-}��c��Cu��N{H}_{3}{��_{2}}^{+}��}{{c}^{2}��N{H}_{3}•{H}_{2}O��}$��$\frac{c��C{u}^{+}��}{c��C{u}^{+}��}$=Ksp��K��
������CuI��s��+2NH3•H2O��aq��?Cu��NH3��2+��aq��+I-��aq��+2H2O��1��������ϵ���㰱ˮ���ʵ�����c=$\frac{n}{V}$��
��� �⣺��1��������ת����ϵ������֪���ƻƣ�As2S3��������Һ�к�Sn2+���ӷ���������ԭ��Ӧ����As4S4��H2S��Sn4+����ϵ���غ㡢ԭ���غ���ƽ��д���ӷ���ʽΪ��2As2S3+4H++2Sn2+=As4S4+2H2S+2Sn4+��
�ʴ�Ϊ��2As2S3+4H++2Sn2+=As4S4+2H2S+2Sn4+��
����1molAs4S4��Ӧת��12mole-����Ӧ����3mol��������Ӧ�ķ���ʽΪ��As4S4+3O2$\frac{\underline{\;һ������\;}}{\;}$2As2O3+4S����aΪS��
�ʴ�Ϊ��S��
��2������Na2HPO4��Һ�м�������CaCl2��Һ��������Һ�����ԣ�˵����Ӧ��������Ƴ������Ȼ��ƺ��Ȼ��⣬��Ӧ�����ӷ���ʽΪ��3Ca2++2HPO42-=Ca3��PO4��2+2H+��
�ʴ�Ϊ��3Ca2++2HPO42-=Ca3��PO4��2+2H+��
��ͼ���п�֪H2PO4-��HPO42-���ʵ�����ͬ������ΪNa2HPO4��NaH2PO4 �����Ԫ���غ����õ�����������������ʵ���֮��2��3��
�ʴ�Ϊ��H2PO4-��HPO42-��2��3��
����֪������������������ɣ������������ӣ�pH��2��4֮�䣬�����δ�������ˮʱ������ҺpH��������Ч����ǿ��ͼ2������֪��PH����H2PO4-�������ͬʱ���ɸ�����������������
�ʴ�Ϊ����PH����H2PO4-�������ͬʱ���ɸ�����������������
��3����Cu+��aq��+2NH3•H2O��aq��?Cu��NH3��2+��aq��+2H2O��1��K=8.0��1010
CuI��s��+2NH3•H2O��aq��?Cu��NH3��2+��aq��+I-��aq��+2H2O��1����
ƽ�ⳣ��K=$\frac{c��{I}^{-}��c��Cu��N{H}_{3}{��_{2}}^{+}��}{{c}^{2}��N{H}_{3}•{H}_{2}O��}$=$\frac{c��{I}^{-}��c��Cu��N{H}_{3}{��_{2}}^{+}��}{{c}^{2}��N{H}_{3}•{H}_{2}O��}$��$\frac{c��C{u}^{+}��}{c��C{u}^{+}��}$=Ksp��K=2.0��10-12��8.0��1010=0.16��
�ʴ�Ϊ��0.16��
���ܽ�19.1g CuI��s�����ʵ���=$\frac{19.1g}{191g/mol}$=0.1mol��CuI��s��+2NH3•H2O��aq��?Cu��NH3��2+��aq��+I-��aq��+2H2O��1������Ҫ�������ʵ���Ϊ0.2mol��������Ҫ5mol•L-1��ˮ�����=$\frac{0.2mol}{5mol/L}$=0.04L=40ml��
�ʴ�Ϊ��40��
���� ���⿼����������ԭ��Ӧ�����ӷ���ʽ��д��ͼ������жϡ�ƽ�ⳣ�������֪ʶ�㣬���ջ���ע����������Ϣ�ǽ���ؼ�����Ŀ�ѶȽϴ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH=5��CH3COOH��Һ��pH=5��NH4Cl��Һ�У�c��H+������� | |
| B�� | pH=8.3��NaHCO3��Һ�У�c��Na+����c��HCO3-����c��CO32-����c��H2CO3�� | |
| C�� | 0.1 mol AgCl��0.1 mol AgI��Ϻ����1 Lˮ�У�������Һ�У�c��Cl-��=c��I-�� | |
| D�� | 0.2 mol/L CH3COOH��Һ��0.1 mol/L NaOH��Һ�������Ϻ�������Һ�У�2c��H+��-2c��OH-��=c��CH3COO-��-c��CH3COOH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �� | B�� | ��ϩ | C�� | ���� | D�� | ʮ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
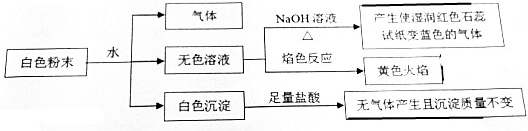
| A�� | һ������BaCl2��Mg��NO3��2��NaHCO3��X����X��NH4HSO4 | |
| B�� | һ������K2CO3��CuSO4��X��NH4Al��SO4��2 | |
| C�� | һ������BaCl2��NaHCO3��X��X��NH4AlO2����һ��ΪNaCl��Mg��NO3��2�е���һ�� | |
| D�� | һ������NaCl��BaCl2��NaHCO3��X����X����ȷ����ʲô���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

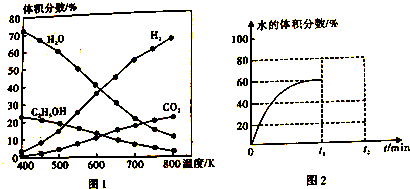
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
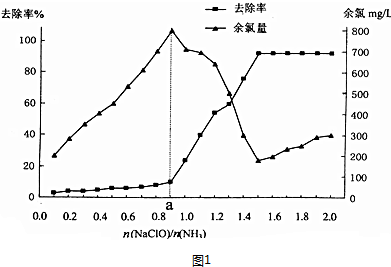
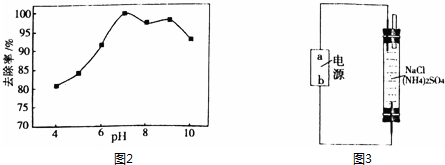
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
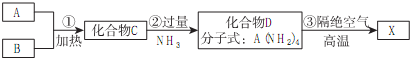

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��Һ��ֻ��Fe3+��SO42-��Cl- | |
| B�� | ��Һ��ֻ��Cu2+��SO42- | |
| C�� | ��Ҫ������ɫ��Ӧ����ȷ������Na+ | |
| D�� | ��Һ�п϶�û��I-������ȷ������Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
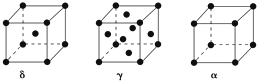
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com