
��ѧ��һ����ʵ��Ϊ���Ŀ�ѧ����ѧʵ����ѧϰ̽���������ʵĻ�������֮һ��
��1�������й�������ȷ����__________����д��ţ�
a��ʹ��������ƽ�ĵ�һ�������ǽ��������������̶ȴ�
b�����˲��������У�Ϊ�ӿ�����ٶȿ��ò�������©���е���Һ���н���
c����Ũ��������ϡ��Һʱ������Ͳ�к�ϡ��Ҫ��ȴ��������ת�Ƶ�����ƿ��
d��������ƿ������Һʱ�����ݺ�ҡ��Һ���½����ټ�����ˮ���̶��ߴ���������ҺŨ��ƫ��
��2�������������壺H2��O2��NH3��SO2��NO2��NO������������ͼ��ʾװ�ý����ռ���
���������B�ڽ��룬���ռ���������_______________��
��������ƿ��ע��ˮ��������Ӧ�ô�___����д��A����B�����ڽ��룬�����ռ���������_____��
��1��ad��2�֣���ѡ����ѡ�����÷֣� ��2����H2��NH3(2��)
��B��2�֣� H2��O2��NO��2�֣���ѡ����ѡ�����÷֣�
���������������1��a��ʹ��������ƽ�ĵ�һ�������ǽ��������������̶ȴ��������ƽ�Ƿ�ƽ�⣬a��ȷ��b�����˲��������У�������ֻ�����������������ò�������©���е���Һ���н��裬b����c����Ͳ��������ϡ����Һ���ܽ���壬������Ũ��������ϡ��Һʱ��Ӧ���ձ���ϡ�ͺ�Ҫ��ȴ��������ת�Ƶ�����ƿ�У�c����ȷ��d��������ƿ������Һʱ�����ݺ�ҡ��Һ���½����ټ�����ˮ���̶��ߴ�������Һ��������ӣ����������ҺŨ��ƫ�ͣ�d��ȷ����ѡad��
��2������������ռ�װ��ʱ������B��ͨ������ʱ���൱�ڲ�ȡ������С�ſ������ռ����壬�ռ��������ܶ�ӦС�ڿ������ܶȣ����������������Ӧ�����Է����������������Ͱ�����
��������ƿ��ע��ˮ��������Ӧ�ô�B�ڽ��룬�����ռ������岻����ˮ��Ӧ��Ҳ��������ˮ��NH3��SO2��NO2������ˮ��Ӧ�����Կ����ռ���������H2��O2��NO��
���㣺���黯ѧʵ�����������������ռ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������ʵ��װ�ý�����Ӧ��ʵ�飬�ܴﵽʵ��Ŀ�ĵ���
| A��ͼI����ʵ�����ư������ռ�����İ��� | B��ͼII���ڳ�ȥCO2�к��е�����HCl |
| C��ͼIII������ȡI2��CCl4��Һ�е�I2 | D��ͼIV���ڼ���ʽ�ζ����Ƿ�©Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣���ͼ��A��B��C��D�dz�������ͨ�����Լ�ƿ����������Լ��������д���ʺ�ʢ�ŵ��Լ�ƿ����������ڣ�
a��Ũ���� b��̼������Һ c����Ƭ d��Ũ���� e������������Һ f����������
| A | B | C | D |
 |  |  |  |
| �� �� | �� �� | �� �� | �� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ϣʱ�������Ĵ������������Ի�������˼������в��ij�����Ϊ����ѧ��̽��С�齫һ����������·������õ���70%Cu��25%Al��4%Fe������Au��Pt�Ƚ����Ļ�������Ƴ������Ʊ�����ͭ�����·�ߣ�
�ش��������⣺
�ŵڢٲ�Cu���ᷴӦ�����ӷ���ʽΪ ���õ�����1����Ҫ�ɷ�Ϊ ��
�Ƶڢڲ�����H2O2�������� ��ʹ��H2O2���ŵ��� ������pH��Ŀ����ʹ ���ɳ�����
���õڢ۲�����CuSO4��5H2O�Ʊ���ˮCuSO4�ķ����� ��
��������2��ȡAl2(SO4)3��18H2O��̽��С����������ַ�����
�������ַ����У� ���������У�ԭ���� ��
��ԭ�������ʽǶȿ��ǣ� ������������
��̽��С���õζ����ⶨCuSO4��5H2O(Mr=250)������ȡa g �������100 mL��Һ��ÿ��ȡ20.00 mL�������������Ӻ���c mol/L EDTA(H2Y2��)����Һ�ζ����յ㣬ƽ������EDTA��Һb mL���ζ���Ӧ���£�Cu2++ H2Y2����CuY2��+ 2H+
д������CuSO4��5H2O���������ı���ʽ�أ� ��
���в����ᵼ��CuSO4��5H2O�����ⶨ���ƫ�ߵ��� ��
a��δ������ƿ
b���ζ��յ�ʱ�ζ��ܼ����в�������
c��δ��������EDTA��Ӧ�ĸ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����п��ĩ�㷺Ӧ������Ϳ�ϡ��մɡ�������ҽҩ����������ҵ��Ϊ�ۺ�Ӧ����Դ������ұ��п��п��Ʒ�ӹ���ҵ���յ�п��������ZnO��FeO��Fe2O3��CuO��Al2O3�����ʣ�����ȡ����п��������ͼ��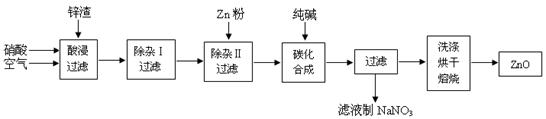
�й��������������ȫ��pH���±���
��1������������У�����п����ɷ�ĩ��ͨ�������ͬʱ����������������� ��
��2���������նദ�漰�����ˡ���ʵ�����й�����Ҫʹ�õIJ����������ձ��� ��
��3�����ڡ����Ӣ��У���������KMnO4��Һ����Ŀ���� ��KMnO4�Ǹ÷�Ӧ�� ������������ԭ����������Һ��pH����4��Ŀ���� ��
���ڡ�����II���У�����п�۵�Ŀ���� ��
��4���ڡ�̼���ϳɡ��У��������м�ʽ̼��п��Zn2(OH)2CO3�ݺ�CO2�ȣ���д���÷�Ӧ�Ļ�ѧ����ʽ ��
��5���������У����˷�������϶࣬�����Ե�ȱ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ظ�����ǹ�ҵ������ʵ���ҵ���Ҫ����������ҵ�ϳ��ø�������Ҫ�ɷ�ΪFeO ? Cr2O3)Ϊԭ��������ʵ����ģ�ҵ���ø�������K2Cr2O7����Ҫ�������£��漰����Ҫ��Ӧ�ǣ�
6FeO��Cr2O3+24NaOH+7KClO3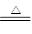 12Na2CrO4+3Fe2O3+7KCl+12H2O
12Na2CrO4+3Fe2O3+7KCl+12H2O
��ش��������⣺
��1���ڷ�Ӧ�����У���Na2CrO4���ɣ�ͬʱFe2O3ת��ΪNaFeO2������SiO2��Ai2O3�봿�Ӧת��Ϊ�������Σ�д����������̼���Ʒ�Ӧ�Ļ�ѧ����ʽ��________________��
��2) NaFeO2��ǿ��ˮ�⣬�ڲ��������ɳ�������ȥ��д���÷�Ӧ�Ļ�ѧ����ʽ��_______________��
��3����Ҫ���������۵�Ŀ�ģ�________________________��
��4���������У��ữʱ��CrO42-ת��ΪCr2O72-��д��ƽ��ת�������ӷ���ʽ��___________��
��5����ȡ�ظ��������2. 5000g���250mL��Һ��ȡ��25.00mL�ڵ���ƿ�У�����10mL2mol/LH2SO4�������⻯�أ����Ļ�ԭ����ΪCr3+)�����ڰ���5min��Ȼ�����100mLˮ������3mL����ָʾ������0.1200mol/LNa2S2O3����Һ�ζ���I2+2S2O32��=2I��+S4O62���������մﵽ�ζ��յ㹲��ȥNa2S2O3����Һ40.00mL�������ò�Ʒ�ظ���صĴ���________________ (�������������������ʲ����뷴Ӧ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʯ�к��е���Ҫ��������������������ij��ѧ��ȤС���ô���ʯΪԭ����ȡ��ȫ��ɱ�����������Ƶ���Ҫ���̣�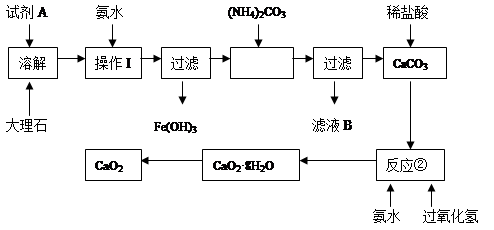
��ش��������⣺
��1���Լ�A�������� ��
��2������I��Ŀ���� ��
��3����ʵ����Ҫ���ʹ�ù��ˣ������С�һ���������͡��������������С����͡�ָ ��
��4��д����Ӧ��������CaO2��8H2O�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��14�֣�ˮ�����л�������нϸߵĻ�ѧ����������������KMnO4�����л�����京������Ҫ�����������£�
��1������AΪ ������ʽ���ʽ���ζ��ܣ�Ҫ˳�����ʵ�飬���������Һ��ɫӦΪ ��
��2�����Ʋ�����漰�����ӷ���ʽ��
C2O42��+ MnO4��+ = Mn2++ CO2��+
�ò����е����һ��Na2C2O4ʱ��ɫ�������Ժ�ĵζ�����ɫ�Ͽ죬��ԭ���� ��
��3���������������Na2C2O4��ҺΪ20.00ml����֪�ζ����Һ����ͼ��ʾ������ͼ�б���ζ�ǰ��Һ�档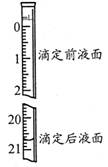
��4����ʵ����������ʵ���л��ﺬ��ƫ�ߣ��ֱ����������������룺
����1��ˮ����Cl��Ӱ��
����2������Na2C2O4��Һ����ʱ ��
��5��������1��������������Ͽ�Ƭ������������ʵ��������Cl��Ӱ�졣����ѡ�Լ���AgNO3��Һ��Ag2SO4��Һ��KMnO4��Һ��Na2C2O4��Һ�� ��
���Ͽ�Ƭ��
1���л��ʿ�HNO3��������
2��AgCl��������KMnO4��Һ��Ӧ��
3��Ag2C2O4�ɱ�����KMnO4��Һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
KMnO4��Һ������������ԭ��Ӧ�ζ��ı�Һ�仹ԭ����ΪMn2+������KMnO4��ǿ�����ԣ�������Һ�����ױ������л�ˮ�е�ijЩ������ԭ�����ʻ�ԭ����������������MnO(OH)2���������KMnO4����Һ�IJ���������ʾ��
�ٳ�ȡ�Զ�����������KMnO4��������ˮ�У�����Һ���Ȳ�������1 h�������ײ���©�����˳�ȥ���ܵ�MnO(OH)2���۹��˵õ�KMnO4��Һ�����棻������������ԭ�ζ���������70��80 ���������û��Լ�(���ȸߡ���Է��������ϴ��ȶ��ԽϺõ�����)��Һ�궨��Ũ�ȡ�
��ش��������⣺
(1)���ƺõ�KMnO4��Һ���淽���� ��ȷ��ȡһ�������KMnO4��Һ��Ҫʹ�õ�������____________��
(2)�����������У����ڱ궨KMnO4��Һ�Ļ��Լ����ѡ��________(�����)��
| A��H2C2O4��2H2O | B��FeSO4 | C��Ũ���� | D��Na2SO3 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com