
Ϊ̽��ij����ҩX����ɣ���������ʵ�飺
�������ϣ�
�ٿ���ҩX���ܵ���ɿ��Ա�ʾΪ��MgmAln(OH)p(CO3)q(SiO3)r��m��n��p��q��rΪ��0����������
��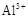 ��pH=5.0ʱ������ȫ��
��pH=5.0ʱ������ȫ�� ��pH=8.8ʱ��ʼ������pH=11.4ʱ������ȫ��
��pH=8.8ʱ��ʼ������pH=11.4ʱ������ȫ��
ʵ����̣�
| ���� | ʵ����� | ʵ������ |
| I | ��X�ķ�ĩ�м���������� | ��������A���õ���ɫ��Һ |
| II | ������õ���Һ�еμӰ�ˮ������pH��5~ 6������ | ���ɰ�ɫ����B |
| III | �����B�мӹ���NaOH��Һ | ����ȫ���ܽ� |
| IV | ��II�õ�����Һ�еμ�NaOH��Һ������pH��12 | ���ɰ�ɫ����C |
��1��CO2
��2��Al3++3NH3��H2O ="=" Al(OH)3��+3NH4+
��3��Al(OH)3+OH��="=" AlO2��+ 2H2O
��4��Mg(OH)2 ��5��Mg3Al2(OH)10CO3
���������������1����X�ķ�ĩ�м�����������������A���õ���ɫ��Һ��˵������CO32-��û��SiO32-�����ܷ����ķ�ӦΪ��CO32-+ 2H+== CO2 �� + H2O��SiO32-��2H+=H2SiO3������������A��ʹ����ʯ��ˮ����ǣ�A�Ļ�ѧʽ��CO2��������õ���Һ�еμӰ�ˮ������pH��5~ 6�����ɰ�ɫ����B������Al3+��pH=5.0ʱ������ȫ��Mg2+��pH=8.8ʱ��ʼ������pH=11.4ʱ������ȫ˵������Al3+.����B��Ӧ�����ӷ���ʽ��Al3++3NH3��H2O ="=" Al(OH)3��+3NH4+.�����B�мӹ���NaOH��Һ������ȫ���ܽ⣬��ʱ������ӦAl(OH)3+OH��="=" AlO2��+ 2H2O����II�õ�����Һ�еμ�NaOH��Һ������pH��12�����ɰ�ɫ����C��˵������Mg2+��Mg2++2NH3��H2O ="=" Mg(OH)2��+2NH4+.����C�Ļ�ѧʽ��Mg(OH)2����5��������n(A)�Un(B)�Un(C)=1�U2�U3��n(CO2):n(Al(OH)3):n(Mg(OH)2)=1��2��3����n(CO32-):n(Al3+):n(Mg2+)=1�U2�U3������n(CO32-)=1mol.�ٸ������ʳʵ����Կ�֪��Ӧ�ú���OH-���ӣ������ʵ���Ϊ10mol����X�Ļ�ѧʽ��Mg3Al2(OH)10CO3��
���㣺�������ӵļ��顢���ӷ�Ӧ����ʽ����д�����ʳɷֵ�ȷ����֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����þ��ҽҩ����������ҵӦ�ù㷺������þ��ԭ�Ƚ��Ʊ��ߴ�����þ��һ���µ�̽��������þ��(��Ҫ�ɷ�Ϊ(MgCO3��������FeCO3)Ϊԭ���Ʊ��ߴ�����þ��ʵ���������£�
��1��ͨ��O2����ʱ��������Ӧ�����ӷ���ʽΪ____________________��
��2������2�ijɷ���_________���ѧʽ��
��3������ͼ�С��������衱Ϊ �����˵Ȳ������õ�MgSO4��7H2O���塣��MgSO4��7H2O����ֱ�Ӽ��� ����ܡ����ܡ����õ���ˮMgSO4���塣
��4����ʱ�����MgCO3����Һ������Mg(OH)2���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5����֪���ָʾ�����������ɫ��pH��Χ�����ʾ��
| pH | < 8.0 | 8.0 ~ 9.6 | > 9.6 |
| ��ɫ | ��ɫ | ��ɫ | ��ɫ |
 2MgO+2SO2��+CO2���� MgSO4+C
2MgO+2SO2��+CO2���� MgSO4+C MgO+SO2��+CO����
MgO+SO2��+CO���� MgO+S��+3CO����
MgO+S��+3CO����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����̿��Ʊ�������ص���Ҫ��Ӧ���£�
�������� 3MnO2+KClO3+6KOH 3K2MnO4+KCl+3H2O
3K2MnO4+KCl+3H2O
�����绯 3K2MnO4+2CO2=2KMnO4+MnO2��+2K2CO3
������ʵ��ܽ�ȣ�293K�����±���
| | K2CO3 | KHCO3 | K2SO4 | KMnO4 |
| �ܽ��/g | 111 | 33.7 | 11.1 | 6.34 |
 2KMnO4+2KOH+H2������ԭ������ȣ���ⷨ������Ϊ ��
2KMnO4+2KOH+H2������ԭ������ȣ���ⷨ������Ϊ ��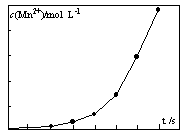
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ�Ǿ����Դ���⣬��ˮ���������ۺ����þ�����Ҫ���塣
���������գ�
��1���ȼҵ��Ҫ��ʳ��Ϊԭ�ϡ�Ϊ�˳�ȥ�����е�Ca2+��Mg2+��SO42������ɳ���ɽ���������ˮ��Ȼ��������в�������ȷ�IJ���˳���� ��
�ٹ��ˣ��ڼӹ�����NaOH��Һ���ۼ�����������ܼӹ�����Na2CO3��Һ���ݼӹ�����BaCl2��Һ
a���ڢݢܢ٢� b���٢ܢڢݢ� c d���ݢڢܢ٢�
��2����ʵ�����п�������ȡ�ķ�����ȡ�壬��ѡ�õ��Լ���________________��������Ҫ������������____________________��
��3����������������ữ�����Cl2�����ʵ�ԭ���� ��
��4������II��Ӧ�����ӷ���ʽ__________________________________________��
��5����ˮ������������У��¶�Ӧ������80~90�棬�¶ȹ�����Ͷ����������� �������ԭ�� ��
��6��Mg(OH)2�����л���Ca(OH)2����ѡ��__________��Һ����ϴ�ӳ�ȥ����ֱ�Ӽ���Mg(OH)2�õ�MgO���ٵ������MgO�ƽ���þ�������ɼ�ʵ�鲽�裬��_______��ѡ�ͬ�⡱������ͬ�⡱����˵���������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ�������������NaI��KCl��Na2CO3��Na2SO4��CaCl2��Cu(NO3)2�е�һ�ֻ�����ɣ�Ϊ�˼������������ʣ���������ʵ�飺
��ȡ������������ˮ���õ���ɫ����Һ��
��������Һ�еμ��Ȼ�����Һ���а�ɫ�������ɣ�
�۹��ˣ��������м���������ϡ���ᣬ���ֳ���û��ȫ���ܽ�������ɫ��ζ���������ɡ�
������Һ�м������������Ƶ���ˮ���ټ����������ͣ������ã��ϲ�Һ����Ϻ�ɫ��
��1�����жϣ����������п϶����� ��
һ��û�� ��
���ܺ��� ��
��2���Կ��ܺ��е����ʣ���ν���ʵ���Խ�һ�����顣
��
��3��ʵ����з����Ļ�ѧ��Ӧ���� ��Ӧ���Ӧ���ͣ�����Ҫʵ��������ƽ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij���������̿�MnO2Լ70����Al2O3������п��ZnSԼ80����FeS������ͬ����MnO2��Zn���ɵ��ԭ�ϣ���
��֪����A��MnSO4��ZnSO4��Fe2��SO4��3��Al2��SO4��3�Ļ��Һ��
��IV�еĵ�ⷴӦʽΪMnSO4+ZnSO4+2H2O  MnO2+Zn+2H2SO4��
MnO2+Zn+2H2SO4��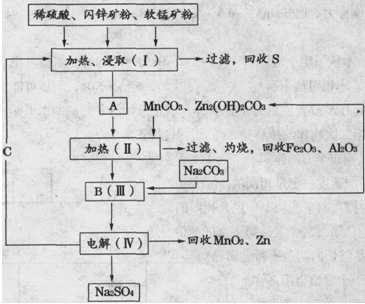
��1��A�����ڻ�ԭ������� ��
��2��MnCO3��Zn2��OH��2CO3�������� ������Ҫ���ȵ�ԭ���� ��C�Ļ�ѧʽ�� ��
��3�����з��������ӷ���ʽΪ �� ��
��4����������������е���ģ�����ʯ�⣬�蹺��Ļ���ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±����������ڲ�ͬ�¶��µ��ܽ�ȣ�g/100gˮ����
| | NaNO3 | KNO3 | NaCl | KCl |
| 10�� | 80.5 | 21.2 | 35.7 | 31.0 |
| 100�� | 175 | 246 | 39.1 | 56.6 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
һ�ֺ�����ﮡ��ܵ����͵��Ӳ��ϣ������в����ķ��������ɹۣ������е����Խ�����������ʽ���ڣ�����Co2O3·CoO����ʽ���ڣ������������ĵ����˫�棻﮻��������С�
�ӷ����л��������ܣ�CoO���Ĺ����������£�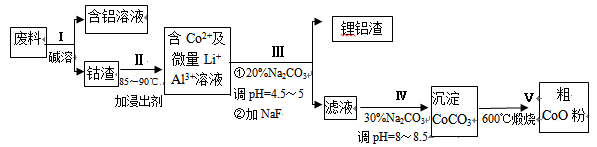
��1������I�в���NaOH��Һ�ܳ������е�Al����Ӧ�����ӷ���ʽΪ ��
��2������II�м���ϡH2SO4�ữ���ټ���Na2S2O3��Һ�����ܡ�������ܵĻ�ѧ��Ӧ����ʽΪ��������ֻ��һ������� ����ʵ����ģ�ҵ����ʱ��Ҳ������������ܣ���ʵ�ʹ�ҵ�����в������ᣬ��ӷ�Ӧԭ������������������ܵ���Ҫԭ��_______________��
��3�����̢�õ����������Ҫ�ɷ���LiF��Al(OH)3��̼������Һ�ڲ���Al(OH)3ʱ����Ҫ���ã���д���÷�Ӧ�����ӷ���ʽ________________________��
��4��̼������Һ�ڹ���III��IV����������������ͬ����д���ڹ���IV�����������
____________________________________________________________��
��5����Na2CO3��Һ�д��ڶ������ӣ����и�����Ũ�ȹ�ϵ��ȷ����______������ţ���
| A��c(Na+) = 2c(CO32-) | B��c(Na+) > c(CO32-) > c(HCO3-) |
| C��c(OH-) > c(HCO3-) > c(H+) | D��c(OH-) - c(H+)��c(HCO3-) + 2c(H2CO3) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ᴿ��������ʵķ����У�
| A����ȡ��Һ�� | B�����ȷֽ⣻ | C�������ᾧ�� | D����Һ��E������F�����˵ȣ��뽫�ᴿ����뷽����������ں�������ϡ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com