
����Ŀ���к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�顣ȡ0.55mol/L��NaOH��Һ50mL��0.25mol/L������50mL����ͼ��ʾ��װ���н����к��ȵIJⶨʵ�飬�ش��������⣺

��1������ͼʵ��װ�ÿ���������ȱ�ٵ�һ�ֲ�����Ʒ��__________________________��
��2��������60mL 0.25mol��L-1H2SO4��50mL 0.55mol��L-1NaOH��Һ���з�Ӧ������ʵ����ȣ����ų�������___________�����ȡ���������ȡ�������ʵ���������ȷ���������к���__________���ȡ�������ȡ�����
��3������NaOH��Һ����ȷ�����ǣ�________�� (������ѡ��)��
A���ز������������롡 B���������������� C��һ��Ѹ�ٵ���
��4��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ�����ǣ�________�� (������ѡ��)��
A�����¶ȼ�С�Ľ��� B���ҿ�ӲֽƬ�ò���������
C����������ձ� D���������¶ȼ��ϵĻ��β������������ؽ���
��5��ʵ���������±���������д�±��еĿհף�
�¶� ʵ������� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ (t2��t1)/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | ______ |
2 | 27.0 | 27.4 | 27.2 | 32.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�ڽ�����Ϊ0.55 mol/L NaOH��Һ��0.25 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c��4.18 J/(g����)�����к�����H��___________ ( ȡС�����һλ)��
������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ�������(����ĸ)_________��
a��ʵ��װ�ñ��¡�����Ч����
b�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
���𰸡����β����������������CD3.4-56.8kJ/mola b c
��������
��1�������ȼƵĹ������֪����װ�õ�ȱ�������ǻ��β������������˱�����������β����������
��2����Ӧ�ų����������������Լ�������Ķ����йأ�����60mL0.25molL-1H2SO4��50mL0.55molL-1NaOH��Һ���з�Ӧ������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ��к�����ȣ���ˣ�������ȷ���ǣ�����ȣ������
��3���к��ȵIJⶨ�У����뾡����������ɢʧ�����Ե�������������Һʱ������һ�β���Ѹ�ٵ����ձ��У�����Cѡ������ȷ�ģ����������C��
��4��A���¶ȼ����ڲⶨ�¶ȣ�����ʹ���¶ȼƽ�����Һ����A����
B���ҿ�ӲֽƬ�ò��������裬�ᵼ������ɢʧ��Ӱ��ⶨ�������B����
C����������ձ����ᵼ����Һ������������������ɢʧ��Ӱ��ⶨ�������C����
D���������¶ȼ��ϵĻ��β���������ؽ���������ʹ���������������Һ��Ͼ��ȣ��ֿ��Լ�������ɢʧ������Dѡ������ȷ�ģ�
�������������������D��
��5�����Ĵβⶨ�¶Ȳ�ֱ�Ϊ3.4�棬5.1�棬3.3�棬3.5�������е�2�ε��¶Ȳ����ϴ�Ӧ�����������������¶Ȳ��ƽ��ֵΪ����3.4+3.3+3.5��/3=3.4������˱�����ǣ�3.4��
��50mL0.55mol/L����������50mL0.25mol/L������Һ�����кͷ�Ӧ������ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025mol����Һ������Ϊ100mL��1g/cm3=100g���¶ȱ仯��ֵΪ��T=3.4����������0.025molˮ�ų�������Ϊ��Q=mc��T=100g��4.18J/��g�棩��3.4��=1421.2J����1.4212KJ������ʵ���õ��к�����H=-1.4212/0.025=-56.8 kJ/mol����ˣ�������ȷ���ǣ�-56.8kJ/mol��
��a��ʵ��װ�ñ��¡�����Ч������ã��������������ʧ���ⶨ���ƫС����a��ȷ��
b���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ���Ϊ�¶ȼ��ϻ����������ƣ��������������ᷴӦ���ȣ������������ʼ�¶�ƫ�ߣ��¶Ȳ�ƫС���ⶨ���ƫС����b��ȷ��
c������һ�ο��ٽ�NaOH��Һ����ʢ�������С�ձ��У��������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�����ᵼ�½϶�����ɢʧ����c��ȷ��
��ˣ�������ȷ���ǣ�abc��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪FeCl3��Һ��KSCN��Һ��Ϻ�����ӦFeCl3+3KSCN![]() Fe��SCN��3+3KCl���ﵽƽ��ı�������������˵����ȷ����
Fe��SCN��3+3KCl���ﵽƽ��ı�������������˵����ȷ����
A. ����Һ�м�������KC1���壬��Һ��ɫ��dz
B. �����¶ȣ�ƽ��һ�������ƶ�
C. ��������KC1�������������FeCl3����ƽ�ⳣ���������仯���ұ仯�����෴
D. ƽ�ⳣ������ʽΪK��c[Fe(SCN)3]��c3(KCl)/[c(FeCl3)��c3(KSCN)]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����֪��TiO2(s)��2Cl2 (g)��2C(s)��TiCl4(l)��2CO(g) ��H����81 kJ��mol��1
2C(s)��O2(g)��2CO(g) ��H����221 kJ��mol��1
д��TiO2��Cl2��Ӧ����TiCl4��O2���Ȼ�ѧ����ʽ��______________________��
��2������Ӧ����㷺�Ľ���������±����������Լ������ĺ������ξ�Ϊ��Ҫ�������¯������ұ��������Ҫ��������������Ҫ��ӦΪ��
Fe2O3(s)+3CO(g) ![]() 2Fe(s)+3CO2(g) ��H = a kJ mol��1
2Fe(s)+3CO2(g) ��H = a kJ mol��1
��֪�� ��Fe2O3(s)+3C(ʯī) = 2Fe(s)+3CO(g) ��H1 = + 489.0 kJ mol��1
��C(ʯī)+CO2(g) = 2CO(g) ��H2 = + 172.5 kJ mol��1
��a =______kJ mol��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���軯����һ����Ҫ�Ļ�������ԭ�ϣ�ͬʱҲ��һ�־綾���ʣ�����Σ�����ཡ����һ��й¶��Ҫ��ʱ������һ�����ͨ������˫��ˮ�������������Һ���������Լ��ỷ����Ⱦ��
��.��֪���軯�ƻ�ѧʽΪNaCN���軯����һ�ְ�ɫ�ᾧ�������綾��������ˮ��ˮ��Һ�ʼ��ԣ���ˮ�������軯�⡣
��1��CN-��CԪ����+2�ۣ�NԪ����-3�ۣ���ǽ�����N____________C ���������������=�����������ʵ��֤����____________________________________��
��2��NaCN��˫��ˮ��������һ����ʽ�κ�һ����ʹʪ���ɫʯ����ֽ���������壬�÷�Ӧ�����ӷ���ʽ��____________________________________��
��.��������ƵĹ�ҵ�Ʊ��ķ�Ӧԭ��Ϊ2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2��ij��ѧ��ȤС��������ԭ��ʵ�����Ʊ���������ƣ�������������������Һ��������軯�Ʒ�ˮ�ܷ����ŷš�
��ʵ��һ��ʵ����ͨ������ͼ��ʾװ���Ʊ�Na2S2O3��
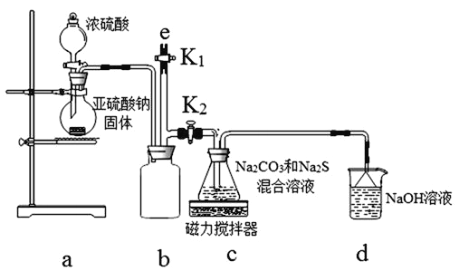
��3��bװ�õ�������________________________��cװ���еIJ�����Na2S2O3��CO2�ȣ�dװ���е�������NaOH��Na2CO3����������__________________________________________________________��
��4��ʵ���������e���������ʢ____________���NaOH��Һ������ˮ������CCl4����һ�֣���ע�������ٹر�K2��K1����ֹ���װ��ʱ��Ⱦ������
��ʵ������ⶨ�������������Һ������ķ�ˮ���軯�Ƶĺ�����
��֪���ٷ�ˮ���軯�Ƶ�����ŷű�Ϊ0.50mg/L��
��Ag++2CN-=[Ag��CN��2]-��Ag++I-=AgI����AgI �ʻ�ɫ���� CN-������ Ag+��Ӧ��
ʵ�����£�ȡ20.00mL��������軯�Ʒ�ˮ����ƿ�У����μӼ���KI��Һ��ָ ʾ������1.00��10-4mol/L�ı�AgNO3��Һ�ζ�������AgNO3��Һ�����Ϊ1.50mL��
��5���ζ��յ������________________________________________________��
��6��������ķ�ˮ�Ƿ�ﵽ�ŷű���____________________________________ ����ǡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E���������Ǻ���Ԫ�صĵ��ʻ������BΪ����ɫ���壬��������ͼ��ʾ��ת����ϵ��
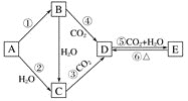
(1)�ƶϸ����������������ʵĻ�ѧʽ��
A____________��B____________��C____________��D____________��E____________��
(2)�ֱ�д����Ӧ�ڡ��ݵĻ�ѧ����ʽ��
��______________________________________��
��__________________________________________��
(3)д����Ӧ�۵����ӷ���ʽ____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��F�Ǽ�ͥ�г������л��E��ʯ�ͻ�����չˮƽ�ı�־��F��һ�ֳ����ĸ߷��Ӳ��ϡ���������ת����ϵ�ش��������⣺

(1)�����ޡ������ߵ����Ʒֱ�Ϊ________��________��
(2)���������зе���ߵ���________��
A ���� B ú��
C ���� D ����
(3)�ڢ١���������ȡ����Ӧ����________��ԭ��������Ϊ100%�ķ�Ӧ��________��(�����)
(4)д���ṹ��ʽ��A________��F________��
(5)д����Ӧ�۵����ӷ���ʽ��___________��
(6)��Ϊ��ͥ�г���������F���������Ǵ����˼���ķ��㣬ͬʱҲ����˻�����Ⱦ��������Ⱦ��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ���ѧ������Ȼ�������о�����ȡ�����½�չ��������ͼװ�ÿɷ�����Ӧ��H2S+O2=H2O2+S����֪�׳��з����ķ�Ӧ��


����˵����ȷ���ǣ� ��
A. �׳���̼���Ϸ����ĵ缫��ӦΪAQ+2H+-2e-=H2AQ
B. �ҳ���Һ�з����ķ�ӦΪH2S+I3-=3I-+S+2H+
C. ��װ���е���ת��Ϊ����
D. H+�Ӽ׳������ҳ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ȤС�������ͼ��ʵ��װ�á�ʵ��ʱ,�ȶϿ�K2,�պ�K1,�����������ݲ���;һ��ʱ���,�Ͽ�K1,�պ�K2,���ֵ�����Aָ��ƫת�������й�������ȷ����

A. �Ͽ�K2,�պ�K1ʱ,ͭ�缫Ϊ����
B. �Ͽ�K2,�պ�K1ʱ,ʯī�缫������Һ���
C. �Ͽ�K1,�պ�K2ʱ,ͭ�缫������ԭ��Ӧ
D. �Ͽ�K1,�պ�K2ʱ,ʯī�缫������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com