
����Ŀ�����Ȼ���������([Co(NH3)6]Cl3)��һ�ֳȻ�ɫ���壬ʵ�����Ʊ��������£�
����ϸ��6 g CoCl26H2O�����4 g NH4Cl���������ƿ�У���ˮ�������ܽ⣬��ȴ��
����13.5 mLŨ��ˮ���û���̿����������Ͼ��Ⱥ���εμ�13.5 mL 5% H2O2��Һ��ˮԡ������50~60�棬����20 min���ñ�ԡ��ȴ�����ˣ��ôֲ�Ʒ��
���ֲ�Ʒ����50 mL�ȵ�ϡ�����У�______������Һ�л�������6.7 mLŨ���ᣬ�д����Ȼ�ɫ������������ԡ��ȴ����ˣ�
�����������2 mol��L1 HCl��Һϴ�Ӿ��壬���������Ҵ�ϴ�ӣ�����ò�Ʒ��
(1)[Co(NH3)6]Cl3��Co�Ļ��ϼ���______��
(2)CoCl2��Ũ��ˮ����Co(OH)2����������Ũ��ˮǰ�ȼ���NH4Cl�ɱ���������ɣ�ԭ����______��
(3)��Һ��CoCl2��NH4Cl��Ũ��ˮ��Ϻ���H2O2��Һ��Ӧ����[Co(NH3)6]Cl3�Ļ�ѧ����ʽ��______��
(4)��ȫ���еIJ�����______��
(5)�����ζ����ⶨ�Ʊ��IJ�Ʒ��Cl��������������
����ȷ��ȡa g ���еIJ�Ʒ�����Ƴ�100 mL��Һ����ȡ25 mL��Һ����ƿ�У�
�����μ�����0.005 mol��L1 K2CrO4��Һ��Ϊָʾ������c mol��L1 AgNO3��Һ�ζ����յ㣻
����ƽ�вⶨ���Σ�����AgNO3��Һ�������ƽ��ֵΪv mL�����㾧����Cl��������������
��֪���ܽ�ȣ�AgCl 1.3��106 mol��L1��Ag2CrO4(ש��ɫ)6.5��105 mol��L1
�٢��У��ζ����յ��������______��
���Ʊ��ľ�����Cl��������������______(�м���ʽ��Cl�����ԭ��������35.5)��
���𰸡�+3 ![]() ����NH3��H2O�ĵ��룬ʹ��Һ�е�c(OH��)���ͣ���������Co(OH)2���� 2CoCl2+10NH3��H2O+2NH4Cl+H2O2
����NH3��H2O�ĵ��룬ʹ��Һ�е�c(OH��)���ͣ���������Co(OH)2���� 2CoCl2+10NH3��H2O+2NH4Cl+H2O2![]() 2[Co(NH3)6]Cl3+12H2O ���ȹ��ˡ���ȴ ��Һ�г���ש��ɫ������������ڲ���ʧ
2[Co(NH3)6]Cl3+12H2O ���ȹ��ˡ���ȴ ��Һ�г���ש��ɫ������������ڲ���ʧ ![]()
��������
�������⣬�ڰ������Ȼ�林��������£��Ի���̿Ϊ��������˫��ˮ����CoCl2��Һ���Ʊ� [Co(NH3)6] Cl3����Ӧ�Ļ�ѧ����ʽΪ2CoCl2+10NH3��H2O+2NH4Cl+H2O2![]() 2[Co(NH3)6]Cl3+12H2O�����ֲ�Ʒ����50 mL�ȵ�ϡ�����У�������Һ�л�������6.7 mLŨ���ᣬ�д����Ȼ�ɫ������������ԡ��ȴ����ˣ�˵�������ȵ�ϡ�����Ӧ���ˣ��������2 mol��L1 HCl��Һϴ�Ӿ��壬���������Ҵ�ϴ�ӣ�����ò�Ʒ��
2[Co(NH3)6]Cl3+12H2O�����ֲ�Ʒ����50 mL�ȵ�ϡ�����У�������Һ�л�������6.7 mLŨ���ᣬ�д����Ȼ�ɫ������������ԡ��ȴ����ˣ�˵�������ȵ�ϡ�����Ӧ���ˣ��������2 mol��L1 HCl��Һϴ�Ӿ��壬���������Ҵ�ϴ�ӣ�����ò�Ʒ��
(1). [Co(NH3)6]Cl3��NH3Ϊ���壬�ȵĻ��ϼ�Ϊ-1�ۣ���Co�Ļ��ϼ���+3�ۣ��ʴ�Ϊ+3��
(2).����NH4Clʹ��Һ�е�![]() Ũ������
Ũ������![]() ����NH3��H2O�ĵ��룬ʹ��Һ�е�c(OH��)���ͣ���������Co(OH)2�������ʴ�Ϊ��
����NH3��H2O�ĵ��룬ʹ��Һ�е�c(OH��)���ͣ���������Co(OH)2�������ʴ�Ϊ��![]() ����NH3��H2O�ĵ��룬ʹ��Һ�е�c(OH��)���ͣ���������Co(OH)2������
����NH3��H2O�ĵ��룬ʹ��Һ�е�c(OH��)���ͣ���������Co(OH)2������
(3).�ɷ�����֪����Һ��CoCl2��NH4Cl��Ũ��ˮ��Ϻ���H2O2��Һ��Ӧ����[Co(NH3)6]Cl3�Ļ�ѧ����ʽ��CoCl2+10NH3��H2O+2NH4Cl+H2O2![]() 2[Co(NH3)6]Cl3+12H2O���ʴ�Ϊ��CoCl2+10NH3��H2O+2NH4Cl+H2O2
2[Co(NH3)6]Cl3+12H2O���ʴ�Ϊ��CoCl2+10NH3��H2O+2NH4Cl+H2O2![]() 2[Co(NH3)6]Cl3+12H2O��
2[Co(NH3)6]Cl3+12H2O��
(4).�ɷ�����֪���ڢ���ȱ�ٲ����ǹ��ˣ��������ڼ����ȵ�ϡ����ʴ�Ϊ�����ȹ��ˡ���ȴ��
(5).�������֪��AgCl���ܽ��С��Ag2CrO4����Һ�ȣ������ڵμ�AgNO3ʱAg+����Cl-��Ӧ����AgCl��������Cl-������ʱ����CrO42-��ϣ����Եζ��յ�ʱ��Ag+��CrO42-�������ש��ɫ���� ���ʴ�Ϊ����Һ�г���ש��ɫ������������ڲ���ʧ��
����AgCl�����Ļ�ѧʽ��֪����ӦʱAg+��Cl-���ʵ���֮��Ϊ1:1���ζ�ʱ����AgNO3�����ʵ���Ϊcmol/L��vmL��10-3=cv��10-3mol����25ml��Һ��Cl-���ʵ���Ϊcv��10-3mol��25ml��Һ��Cl-������Ϊ��m=n��M=cv��10-3mol��35.5g/mol=35.5 cv��10-3g����100g��Һ��Cl-������Ϊ��4��35.5 cv��10-3g������������Ϊ��![]() ���ʴ�Ϊ
���ʴ�Ϊ![]() ��
��


 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ȼ���ͭ����Ҫ��ͭ��ϵ�л�����Ʒ���㷺Ӧ����ʯ�ͻ������л��ϳɵ���ҵ��CuCl����ʰ�ɫ�������ֽ⣬����ˮ��������ϡ������Ҵ���¶���ڳ�ʪ��������ˮ������Ϊ��ɫ��![]() ��ij�о�С����
��ij�о�С����![]() (������
(������![]() )��ƷΪԭ����ȡCuCl����Ƶĺϳ�·�����£�
)��ƷΪԭ����ȡCuCl����Ƶĺϳ�·�����£�
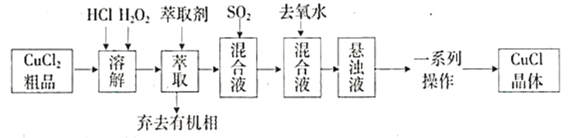
��֪�����ڽϸ�Ũ�ȵ������£�![]() ���ܽ��ڼ��춡����ͪ��
���ܽ��ڼ��춡����ͪ��
��CuCl����Һ�д��ڣ�![]()
(1)�����ϳ�·���У�![]() ��������________________________����ȡ��Ϊ���춡����ͪ����������________________��
��������________________________����ȡ��Ϊ���춡����ͪ����������________________��
(2)�����ϳ�·���У�![]() ͨ����Һ��ʵ��װ����ͼ��ʾ��
ͨ����Һ��ʵ��װ����ͼ��ʾ��
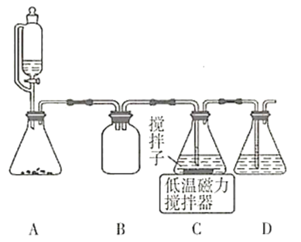
��װ��B��D�����÷ֱ���________________________________________________��
��C�з�Ӧ�����ӷ���ʽ��___________________________________��
(3)�����ϳ�·���У�����Һ�м������ȥ��ˮ��Ŀ����______________________��
(4)�����ϳ�·���У�һϵ�в������������ˡ�ϴ�ӡ����
����ʱӦע���ܷ⡢_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����֪��Al2O3(s)��3C(s)=2Al(s)��3CO(g) ��H1����1 344.1 kJ��mol��1��2AlCl3(g)=2Al(s)��3Cl2(g) ��H2����1 169.2 kJ��mol��1����Al2O3��C��Cl2��Ӧ����AlCl3���Ȼ�ѧ����ʽΪ______________��
��2������Ͻ�ThNi5�ɴ���CO��H2�ϳ�CH4 �ķ�Ӧ����֪�¶�ΪTʱ��CH4(g)��2H2O(g)=CO2(g)��4H2(g)����H����165 kJ��mol��1��CO(g)��H2O(g)=CO2(g)��H2(g)����H����41 kJ��mol��1���¶�ΪTʱ���÷�Ӧ���Ȼ�ѧ����ʽΪ__________��
��3��FeSO4��ת��ΪFeCO3��FeCO3�ڿ����м��ȷ�Ӧ���Ƶ���ϵ��������ϡ���֪25 �棬101 kPaʱ��4Fe(s)��3O2(g)=2Fe2O3(s)����H����1 648 kJ��mol��1��C(s)��O2(g)=CO2(g)����H����393 kJ��mol��1 2Fe(s)��2C(s)��3O2(g)=2FeCO3(s)����H����1 480 kJ��mol��1��FeCO3�ڿ����м��ȷ�Ӧ����Fe2O3���Ȼ�ѧ����ʽ��______��
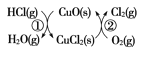
��4����O2��HClת��ΪCl2�������Ч�棬������Ⱦ�� ��ͳ�ϸ�ת��ͨ����ͼ��ʾ�Ĵ�ѭ��ʵ�֣����У���Ӧ��Ϊ��2HCl(g)��CuO(s)![]() H2O(g)��CuCl2(s)����H1����Ӧ������1 mol Cl2(g)�ķ�Ӧ��Ϊ��H2�����ܷ�Ӧ���Ȼ�ѧ����ʽΪ________����Ӧ������H1����H2��ʾ����
H2O(g)��CuCl2(s)����H1����Ӧ������1 mol Cl2(g)�ķ�Ӧ��Ϊ��H2�����ܷ�Ӧ���Ȼ�ѧ����ʽΪ________����Ӧ������H1����H2��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������͡��ۺ�����ж��صĽṹ�������˼��������й㷺��Ӧ�á���ͼ��ij�����͡��ۺ�����Ʊ����̡�
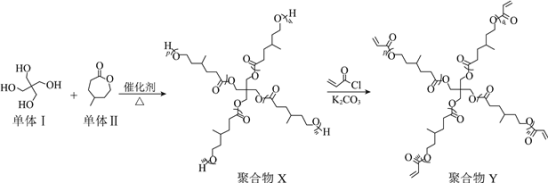
��֪��![]()
����˵������ȷ����
A.�����ĺ˴Ź����������������շ壬�������Ϊ1��2
B.������뵥����Ʊ��ۺ���X����������H2O
C.�ۺ���Xת��Ϊ�ۺ���Y����ȡ����Ӧ
D.�ۺ���Y��ͨ��ĩ�˵�̼̼˫�������γ���״�ṹ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ��������þ���Ͻ�Ͷ��![]() һ��Ũ�ȵ������У��Ͻ���ȫ�ܽ�����������Һ�еμ�Ũ��Ϊ
һ��Ũ�ȵ������У��Ͻ���ȫ�ܽ�����������Һ�еμ�Ũ��Ϊ![]() ��
��![]() ��Һ�����ɵij����������
��Һ�����ɵij����������![]() ��Һ�������ϵ��ͼ���������������λ��
��Һ�������ϵ��ͼ���������������λ��![]() ��������������λ��g����
���������������g����
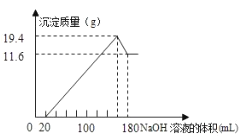
��1���Ͻ���![]() ��
��![]() ������_____________
������_____________
��2������![]() �����ʵ���Ũ��___________
�����ʵ���Ũ��___________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�����ֵ�������й�������ȷ����(����)
A. 14 g��ϩ�ͱ�ϩ��������е���ԭ����Ϊ2NA
B. 1 mol N2��4 mol H2��Ӧ���ɵ�NH3������Ϊ2NA
C. 1 mol Fe���ڹ������ᣬ����ת����Ϊ2NA
D. ��״���£�2.24 L CCl4���еĹ��ۼ���Ϊ0.4NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������һ����Ҫ�Ļ�����Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�ij��ѧС����ʵ�����Ʊ���������������ʵ�顣
��1��ʵ��һ������ͼ��ʾװ���Ʊ����ռ� NH3��
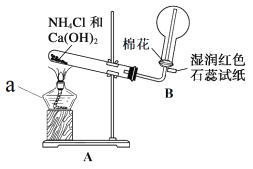
���� a ������Ϊ____________��װ�� A �в��� NH3 �Ļ�ѧ����ʽΪ____________�� װ�� B ����Բ����ƿ�ռ� NH3 �ķ���Ϊ____________��������ſ������������� �ſ�������������ʪ���ɫʯ����ֽ��ɫ��Ϊ____________ɫ����ʾ�Ѿ��ռ��� NH3��
��2��ʵ���������ͼ��ʾװ�ý��� NH3 ����Ȫʵ�顣
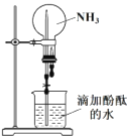
��������Ȫʵ��IJ�������____________���ٴ�ֹˮ�У�ʹ�ձ��ڵ�ˮͨ�����ܽ�����ƿ�γ���Ȫ��˵�� NH3 ���е�����������____________����ƿ����Һ�ʺ�ɫ�� ˵�� NH3 ��ˮ��Һ��____________�ԡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʯ����Ҫ�ɷ���K2SO4��Al2(SO4)3��2Al2O3��6H2O��������������������������ʯ�Ʊ�K2SO4�Ĺ���������ͼ��ʾ��
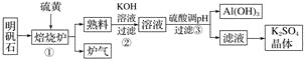
�ݴ�����ͼ�ش��������⣺
(1)�ٱ���¯��Al2(SO4)3��S��Ӧ�IJ���������������÷�Ӧ�Ļ�ѧ����ʽΪ______��д��¯����һ����;________��
(2)����Al2O3���뷴Ӧ�����ӷ���ʽΪ__________��
(3)�����pH������CO2��������____________________��
(4)��ҵұ����������Ҫ���ڸ�����������̼���ԭ����__________________��
(5)��������������������__________________(�Ӧ����)���Ƶ�������Al��NiO(OH)Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ��ŵ�ʱNiO(OH)ת��ΪNi(OH)2���õ�ظ����ĵ缫��Ӧʽ��__________________��
(6)��������ʯ1 625 t�������������Ƶ�780 t Al(OH)3��������Ԫ�ص���ʧ�����������ʯ�м�Ԫ�ص���������Ϊ____________%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ��ͨ������SiO2 ��̼��Ӧ����ȡ�裬д����Ӧ�Ļ�ѧ����ʽ___________________��
��ҵ�ϻ���������þ��ȡ�裬��ӦΪ2Mg+SiO2 = 2MgO+Si��ͬʱ�ᷢ������Ӧ��2Mg + Si = Mg2Si����ͼ�ǽ���Mg��SiO2��Ӧ��ʵ��װ�ã��Իش��������⣺

��1������O2��H2O��g���Ĵ��ڶԸ�ʵ���нϴ�Ӱ�죬ʵ����Ӧͨ������X��Ϊ���������Թ��еĹ���ҩƷ��ѡ��________(�����)��
a��ʯ��ʯ������b��п��������c������
��2��ʵ�鿪ʼʱ��������ͨһ��ʱ��X���壬�ټ��ȷ�Ӧ��������� ___________________________������Ӧ���������߾ƾ��ƣ���Ӧ�ܼ������У���ԭ����______________________��
��3����Ӧ��������ȴ������ʱ������Ӧ��Ļ�����м���ϡ���ᣬ�ɹ۲쵽�����Ļ��ǣ������������ԭ���Ǹ�����Mg2Si������Ѹ�ٷ�Ӧ����SiH4�����飩���壬Ȼ��SiH4��ȼ���û�ѧ����ʽ��ʾ��������Ӧ��________________________��___________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com