
����Ŀ���ڱ�״���½��мס��ҡ�������ʵ�飺�����ȡ30.0 mLͬŨ�ȵ�������Һ������ͬһ��þ���Ͻ��ĩ���������壬�й������б����£�
ʵ����� | �� | �� | �� |
�Ͻ�����/mg | 255 | 385 | 459 |
�����������/mL | 280 | 336 | 336 |
��ش�
(1)����ʵ���У�����______________��ѡ���������������������������ͬ����������______________��Ҫ�����������ʵ���Ũ�ȣ����п����������ݵ�������______________����õ���������ʵ���Ũ��Ϊ______________��
(2)��Ͻ���Mg��Al�����ʵ���֮�ȣ����п����������ݵ�������______________����õ�Mg��Al�����ʵ���֮��Ϊ______________��
(3)�ڱ���ʵ��֮���������м���1.00 mol��L��1 NaOH��Һ����ʹ�Ͻ��е���ǡ���ܽ⣬���γ����ij�������ʹMg2���պó�����ȫ���ٹ��˳������Թ��壬����Һ�и����ʵ����ʵ�����������NaOH��Һ�������д������̣���___________________
���𰸡����� ͬ����������ʱ����H2���� 336mL�����30mL��Һ 1mol/L 255mg��280mL 1��1 NaCl 0.03mol NaAlO2 0.009mol 39 mL
��������
(1)����ʵ��֪�����ӺϽ�������������������˵����ʵ�����������ʣ�ࡣ��Ϊ������ͬ����������ʱ����H2���١�
�Ƚ��Һͱ�ʵ�飬����������ͬ��˵����ʵ�������Ѿ���ȫ��Ӧ������336mL�����30mL��Һ�Ǽ������Ũ������ʹ�õ����ݡ���Ϊ336mL�����30mL��Һ��
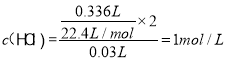 ��Ϊ1mol/L��
��Ϊ1mol/L��
(2)�Ƚϼס���ʵ�����ݿ�֪���������������Ͻ���ȫ��Ӧ������255mg��280mL�Ǽ���Ͻ���Mg��Al�����ʵ���֮������ʹ�õ����ݡ���Ϊ255mg��280mL��
��Mg�����ʵ���Ϊx��Al�����ʵ���Ϊy���з�����Ϊ��
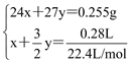 �����x��y��1��1����Ϊ1��1��
�����x��y��1��1����Ϊ1��1��
(3)����Cl-�غ㣬�ɵã�n(NaCl)��n(HCl)��1mol/L��0.03L��0.03mol��
����Al�غ㣬�ɵã�n(NaAlO2)��n(Al)�� ��0.009 mol��
��0.009 mol��
����Na���غ㣬�ɵã�n(NaOH)��0.03mol��0.009mol��0.039mol������V(NaOH)��39 mL��
����NaCl 0.03mol NaAlO2 0.009mol V(NaOH)��39 mL��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£�ˮ�������µ��룺H2O![]() H++OH- H>0������������ȷ����
H++OH- H>0������������ȷ����
A.��ˮ�е�������ϡ���ᣬƽ�������ƶ���Kw��С
B.��ˮ���ȣ�Kw����pH��С
C.��ˮ�м�����������NH4Cl��ƽ�������ƶ���c(H+)����
D.��ˮ�м����������������ƣ�c(H+)=10-7mol/L��Kw����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���0.1mol/L������ͨ���Ȼ������壬������������ȷ���ǣ� ��
A. pH��СB. c(H+)����
C. ˮ�ĵ���̶Ȼ������D. Kw����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ʵ����ͬһԭ�����͵���
A.���Ƶİ�ˮ����ˮ����ʱ��Ͼö�����
B.![]() ��ʹ��ˮ��Ʒ����Һ��ɫ
��ʹ��ˮ��Ʒ����Һ��ɫ
C.![]() ��
��![]() ��Һʹ���Ը��������Һ����ɫ��ȥ
��Һʹ���Ը��������Һ����ɫ��ȥ
D.Ũ�����Ũ���᳤�ڱ�¶�ڿ�����Ũ�Ƚ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о���ѧϰС��Ϊ֤����ͬ��ͬѹ�£���ͬŨ����ͬ��������Բ�ͬ��һԪ��������þ����Ӧʱ�����������������ͬ����Ӧ���ʲ�ͬ��ͬʱ�ⶨʵ���������µ�����Ħ���������Ƶļ���ʵ��װ������ͼ����ʵ�����Ҫ�����������£�

������Ũ�Ⱦ�Ϊ1 mol/L����ʹ�����Һ��
����______________��ȡ10.00 mL 1mol/L����ʹ�����Һ�ֱ����������ƿ�У�
�۷ֱ��ȡ��ȥ��������Ĥ��þ��a g����ϵ��ͭ˿ĩ�ˣ�a����ֵ����Ϊ______________��
���ڹ��ƿ��װ������ˮ����ͼ���Ӻ�װ�ã����װ�õ������ԣ�
�ݽ�ͭ˿�����ƶ���ʹ����þ���������У�ͭ˿������Ӵ���������Ӧ��ȫ����¼______________��
��Ӧ�������¶Ȼָ������£�������Һ���������Һ�棬��ȡ��Ͳ��ˮ�����ǰ��Ӧ______________��������Ͳ��ˮ�����ΪV mL��
�뽫�������貹���������ش��������⣺
�������ֱ����ܼ���װ�������ԵIJ�����۲췽����______________��
�Ʊ�ʵ����Ӧѡ��______________������ţ�����Ͳ��
A��100 mL B��200 mL C��500 mL
����ˮ������Ӱ����Բ��ƣ���ʵ���������£�����Ħ������ļ���ʽΪ��Vm��______________��
�ȼ������ʲ��ȵ�ԭ��______________ͭ˿������Ӵ���ԭ��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.����Ű�Ҫ��ش��������⣺
��1�����и������ʣ���H2O��NH3��KOH��Na2O2��MgCl2��Ne�������ڻ�ѧ������________��ֻ�������Ӽ�����________�����зǼ��Լ������ӻ��������________��
��2�����б仯���̣��ٵ��������NaCl��������ˮ��O2����ˮ��HCl��������ˮ���ռ��ۻ����Ȼ�����ȷֽ⣬��ѧ��û�б��ƻ�����________�����ƻ����Ӽ�����________�����ƻ����ۼ�����________�����ƻ����Ӽ������ƻ����ۼ�����________��
��3��Na2O2�ĵ���ʽΪ________________________���õ���ʽ��ʾH2O���γɹ��̣�________________________________________________��
��.��A��B��C��D��E����������֪��
�ٵ�A��ʧȥ3�����Ӻ�����Ӳ�ṹ����ԭ����ͬ��
�ڵ�B���õ�1�����Ӻ�����Ӳ�ṹ���ԭ����ͬ��
��C����������λ����ɣ��˵����Ϊ12��
��D����18�����ӣ���ʧȥ2�����Ӻ��Ե����ԣ�
��E�������磬ԭ�Ӻ���ֻ��һ�����ӡ���ش�
��1��д�����������ķ��ţ�
A______��B______��C______��D______��E______��
��2��B���Ľṹʾ��ͼΪ____________��C���Ľṹʾ��ͼΪ____________��
��3��A�ĵ�����EB��Һ��Ӧ�����ӷ���ʽΪ______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.���ʵ����ͺ���Ԫ�صĻ��ϼ����о��������ʵ����������ӽǡ��������ͼ��ʾ���ش��������⣺

��1��X�Ļ�ѧʽΪ____________��
��2��W��Ũ��Һ��ͭ�����ڼ��������¿��Է�����ѧ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ______________________��
��3����֪SO3��һ�������������SO3��NaOH��Һ��Ӧ�����ӷ���ʽΪ__________________________________��
��4�����Ʊ�Na2S2O3����������ƣ�����������ԭ��Ӧ�Ƕȷ�������������_______������ţ���
A��Na2S��S B��Na2SO3��S C��Na2SO3��Na2SO4 D��SO2��Na2SO4
��5����X��Y��ϣ������ɵ���ɫ���壬�÷�Ӧ�����������뻹ԭ���������֮��Ϊ_____��
��.��ͼ��ʾ��ͼ��ÿһ�����ʾ�йص�һ�ַ�Ӧ������������A��CΪ��ɫ���壬����д���пհף�
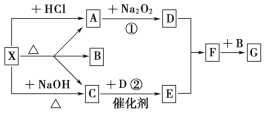
��1������X������_____________��C��______��F��_______��
��2����Ӧ�ٵĻ�ѧ����ʽ��________________________________________����Ӧ�ڵĻ�ѧ����ʽ��___________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����(��)
A. ԭ�Ӻ�������Ų�ʽΪ1s2��ԭ�����������Ų�ʽΪ1s22s2��ԭ�ӻ�ѧ��������
B. Fe3+�����������Ų�ʽΪ3s23p63d5
C. ��̬ͭԭ�ӵ������ĵ����Ų�ͼ: ![]()
D. ��̬̼ԭ�ӵ����������Ų�ͼ:![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�����ϳɾ��ж��ؽṹ�Ļ�����1��2�����ֻ�����1���Ⱥ�ɵû�����2�����¹��ڻ�����1��2��˵������ȷ����( )
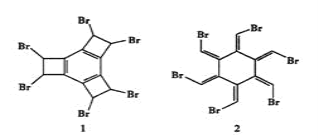
A. ������1��2��Ϊͬ���칹��
B. ������1������ԭ�Ӵ���ͬһƽ��
C. ������1��2�����ڷ����廯����
D. 1 mol������2��ȫȼ������O213.5 mol (Brȼ������![]() HBr)
HBr)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com