
���Ȼ�����S2C12���ǹ㷺������ҵ����������ӽṹ����ͼ��ʾ�������£�S2C12��һ�ֳȻ�ɫҺ�壬��ˮ��ˮ�⣬��������ʹƷ����ɫ�����壬��ѧ����ʽΪ��2S2C12+2H2O��SO2��+3S��+4HCl������˵���д������
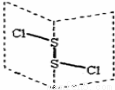
A��S2C12�ĽṹʽΪCl��S��S��Cl
B����Ӧ��SO2�ǻ�ԭ���S����������
C��S2C12Ϊ���м��Լ��ͷǼ��Լ��ķ���
D����Ӧ�У�����1molSO2��ת�Ƶ���Ϊ3mol

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ɳ�и߶��ϵ�һ��˫������ѧ���������棩 ���ͣ�ѡ����
���������Ȼ�ѧ����ʽ�ó��Ľ�����ȷ���ǣ� ��
A����֪2H2(g)+O2(g)=2H2O(g) ��H=-483.6 kJ��mol-��1����������ȼ����Ϊ241.8 kJ��mol��1
B����֪2C(s)+2O2(g)=2CO2(g) ��H=a��2C(s)+O2(g)=2CO(g) ��H=b����a��b
C����֪NaOH(aq)+HCl(aq)=NaCl(aq)+H2O(l) ��H=-57.3 kJ��mol��1����40.0 g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�������С��57.3 kJ
D����֪P (���ף�s)=P (���ף�s) ��H��0������ױȺ����ȶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶���9���¿���ѧ���������棩 ���ͣ������
��ϩ�棨CH2=CHCN����һ����Ҫ�Ļ���ԭ�ϣ���ҵ�Ͽ��á���ϩ������������������Ҫ�������б�ϩȩ��CH2=CHCHO�������棨CH3CN���ȣ��ش��������⣺
��1���Ա�ϩ����������Ϊԭ�ϣ��ڴ������������ɱ�ϩ�棨C3H3N���������ϩȩ��C3H4O���� �Ȼ�ѧ����ʽ���£�
�Ȼ�ѧ����ʽ���£�
��C3H6(g)+NH3(g)+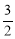 O2(g)=C3H3N(g)+3H2O(g) ��H=��515kJ/mol
O2(g)=C3H3N(g)+3H2O(g) ��H=��515kJ/mol
��C3H6(g)+ O2(g)=C3H4O(g)+H2O(g) ��H=��353kJ/mol
������Ӧ������ѧ�����ƾ��ܴ���ԭ����________________����������߱�ϩ��ƽ����ʵķ�Ӧ������_____________����߱�ϩ�淴Ӧѡ���ԵĹؼ�������___________��
��2��ͼ��a��Ϊ��ϩ������뷴Ӧ�¶ȵĹ�ϵ���ߣ���߲��ʶ�Ӧ�¶�Ϊ460�档����460��ʱ����ϩ��IJ���________________����ǡ����ߡ����ǡ�����Ӧ�¶��µ�ƽ��
���ʣ��ж�������______________������460��ʱ����ϩ����ʽ��͵Ŀ���ԭ����_____________��˫ѡ�����ţ�
A���������Խ��� B��ƽ�ⳣ����� C������Ӧ���� D����Ӧ�������

��3����ϩ��ͱ�ϩȩ�IJ�����n������/n����ϩ���Ĺ�ϵ��ͼ��b����ʾ����ͼ��֪�����n������/n����ϩ��ԼΪ ��������_________________��������������������ϩ�����������ԼΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶���9���¿���ѧ���������棩 ���ͣ�ѡ����
�����������ɷ������·�Ӧ��P4��5O2 == P4O10����֪�������л�ѧ����Ҫ���յ������ֱ�Ϊ��P��P akJ��mol��1��P��O b kJ��mol��1��P��O c kJ��mol��1��O��O d kJ��mol��1������ͼʾ�ķ��� �ṹ���й����ݹ���÷�Ӧ�Ħ�H��������ȷ����( )
�ṹ���й����ݹ���÷�Ӧ�Ħ�H��������ȷ����( )

A��(6a��5d��4c��12b)kJ��mol��1 B��(4c��12b��6a��5d)kJ��mol��1
C��(4c��12b��4a��5d)kJ��mol��1 D��(4a��5d��4c��12b)kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������ѧ��9���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
ij����ﺬ���Ȼ��ơ�̼���ƺ��Ȼ��ء�������֪�����ȵ���������Ϊ35.5%,��û������̼���Ƶ�������������Ϊ
A�� 20% B��30% C��45% D��55%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������ѧ��9���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����ȷ��ʾ���з�Ӧ�����ӷ���ʽ��
A��Fe3O4��������ϡHNO3��Fe3O4��8H��=Fe2����2Fe3����4H2O
B��NH4HCO3��Һ������Ba(OH)2��Һ��ϣ�HCO3����Ba2����OH��=BaCO3����H2O
C�����Ƶ�ˮ�ⷴӦ��S2����H3O�� HS����H2O
HS����H2O
D����0.2 mol��L��1��NH4Al(SO4)2��Һ��0.3 mol��L��1��Ba(OH)2��Һ�������ϣ�2Al3����3SO42����3Ba2����6OH��=2Al(OH)3����3BaSO4��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������ѧ��9���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����������ȷ����
A����5.85 g NaCl��������100 mLˮ�У��Ƶ�0.1 mol/L NaCl��Һ
B����1���c mol/L������Һ��ˮϡ��Ϊ5������õ�0.2c mol/L������Һ
C����25 g��ˮCuSO4����ˮ�Ƴ�100 mL��Һ����Ũ��Ϊ1 mol/L
D����w g a% NaCl��Һ������w/2 gˮ���õ�4a%NaCl��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��1 mol N2��3 mol H2��������ɱ�ĺ����ܱ������У���380���·�����Ӧ��N2(g)+ 3H2{g)  2NH3(g)
2NH3(g)
ƽ��ʱ����ϵ�а������������(NH3)��ѹǿ�仯��������±���

����˵����ȷ���ǣ� ��
A��10MPaʱ��H2��ת����Ϊ75%
B��20 MPaʱ��NH3�����ʵ���Ũ����10MPaʱ��1.5��
C��40 MPaʱ�������������ΪVL����ƽ�ⳣ��
D��30 MPaʱ�����������г���������壬��ƽ��������Ӧ�����ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ�߶��ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��NA��������٤��������ֵ��������������ȷ����
A��H2O2��MnO2����FeCl3�����ķֽⷴӦ�У�ÿ����22.4L������ת��NA����
B��44gCO2��N2O��ɵĻ�����庬�е�������Ϊ22NA
C��1molNa2O2��NA��H2O2�к��еķǼ��Լ�����ΪNA
D��18gH2O��18gNH4+�к��еĵ�������Ϊ10NA
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com