
(I) ��ͼ������ijѧУ����ʦ�Ʊ�NH3 ����������ʵ��ʱ�ĸĽ�װ�á���ͼ�װ�������װ�ã���ȡ2g �����Ȼ��װ���Թܵײ����ٿ��ٳ�ȡ2g �������Ƹ������Ȼ���Ϸ��������ô��еιܵ������������ι�Ԥ������Լ2mL Ũ��ˮ�����ձ���ʢ���з�̪��Һ��ˮ����Ũ��ˮ�����Թ���������۲쵽�Թ��ڷ������ҷ�Ӧ���д�������.

������������NH3 ��Բ����ƿȡ�£���װ��ͼ����ʾ��װ�ã���ͷ�ι�������Ԥ����2mLH2O ����ʱС����ϵ�ڲ������ϳ���Ȼ�ɳ�״̬�����ι��ڵ�ˮ����������ƿ�У�����ζ���ƿ��ͨ���۲�ʵ������������֤NH3 ��ij�����ʡ���Ҫ��ش��������⣺
(1)��ѧ��ѧ�̲�����������O2��ͬ���Ʊ�װ����������ȡNH3�ģ��û�ѧ����ʽΪ:__________________________________________________________
(2)������ijͬѧ��������ʦ����ͼ����ȡNH3��ԭ��������е�������__________��
����NH3 �� H2O ����ƽ��![]() ʹƽ�������ƶ�
ʹƽ�������ƶ�
����NH3�� H2O ����ƽ��![]() ʹƽ�������ƶ�
ʹƽ�������ƶ�
��Na0H ����ˮʱ���ȣ�ʹ��ϵ���¶����ߣ�NH3 ���ܽ�ȼ�С
��NH4Cl��NaOH �ڴ�����¿ɷ�Ӧ����NH3��![]()
��NH4Cl ��ֽ��ͷų�NH3
(3)ͼ���е�NH4Cl ��NaOH ���������ܷ���CaO ������� (��ܡ��롱���ܡ�)
(4)����ж�ͼ������ƿ������NH3 ?_____________________________________________
(5)ͼ���н�ͷ�ι��е�ˮ������ƿ�۲쵽�������� ��˵����NH3

(��)����ͼ��ʾ����B����װ��500 mLˮ���ݻ�Ϊa mL���Թ�A������NO2��NO�Ļ�����壨��״���������Թ�A������B�۵�ˮ�С���ַ�Ӧ���Թ�A��������������Ϊ0.5a mL����ԭ���������NO2��NO�����ʵ���֮��Ϊ
ͨ��������C������0.5a mL������Թ�A�г���ͨ��������A�п��ܹ۲쵽�������ǣ�
___________________________________________________
�йط�Ӧ�Ļ�ѧ����ʽΪ��___________________________________________
���Թ�A�г�������ʱֹͣͨ��������Ȼ���Թ�ȡ��ˮ�ۣ���ͨ�����������Ϊ ________mL��ˮ��B����Һ�����ʵ���Ũ��Ϊ mol��L-1(����Һ�������Ϊ500 mL)
��20�֣�
����1��![]() ��2�֣�
��2�֣�
��2���٢ڢۢ� ��2�֣� ��3���� ��1�֣� ��4�� �ձ��ڵ���Һ��죨1�֣�
��5���������1�֣�����������ˮ��1�֣�
����1��3��1 ��2�֣�
��2����ɫ�����Ϊ����ɫ���壨1�֣����Թ���Һ�治��������ȫ������1�֣�������ͨ���������Թ���Һ���½���1�֣�����������ɫ���壨1�֣���[2NO��O2��2NO2��1�֣� 3NO2��H2O��2HNO3��NO ��1�֣�]�� 4NO��3O2��2H2O��4HNO3 ��2�֣���3��1.375a ��2�֣� a/11200��2�֣�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ÿ��2�֣���22�֣�
I.ʵ����0.01mol/L��KMnO4��������Һ��0.1mol/L��H2C2O4��Һ�������Ϻ�Ӧ���ʦ�[mol/(L �� s)]�뷴Ӧʱ��t��s���Ĺ�ϵ��ͼ��ʾ���ش��������⣺
��1���÷�Ӧ�Ļ�ѧ����ʽ��
��2��0��t2ʱ����ڷ�Ӧ���������ԭ���ǣ� ��
��3��t2��tʱ����ڷ�Ӧ���ʼ�С��ԭ���ǣ� ��
��4��ͼ����Ӱ���֡��������ʾt1��t3ʱ���� ��
����Mn2+���ʵ���Ũ�ȵ����� B��Mn2+���ʵ���������
C��SO42-���ʵ���Ũ�� D��MnO4-���ʵ���Ũ�ȵļ�С
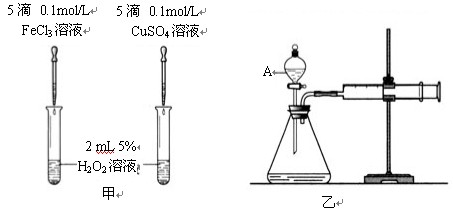
II. Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ����������ͼ�ס�����ʾ��ʵ�顣��ش�������⣺
��1�����Է���������ͼ�ɹ۲� �����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ�������������� ��
��2����������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ ��ʵ������Ҫ������������
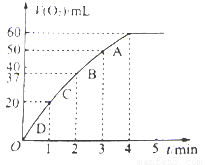
��3������0.01mol MnO2��ĩ��60mL H2O2��Һ�У��ڱ�״���·ų����������ʱ��Ĺ�ϵ��ͼ��ʾ����ų�����������ΪV mL��
�ٷų�V/3 mL����ʱ����ʱ��Ϊ min��
�� ��H2O2��Һ��Ũ��Ϊ
��A��B��C��D���㷴Ӧ���ʿ�����˳��Ϊ > > >
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���ӱ�ʡ������ѧ10-11ѧ���һ��ѧ����ĩ���ԣ���ѧ����B�� ���ͣ�ʵ����
��ÿ��2�֣���22�֣�
I.ʵ����0.01mol/L��KMnO4��������Һ��0.1mol/L��H2C2O4��Һ�������Ϻ�Ӧ���ʦ�[mol/(L �� s)]�뷴Ӧʱ��t��s���Ĺ�ϵ��ͼ��ʾ���ش��������⣺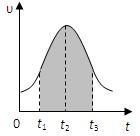
��1���÷�Ӧ�Ļ�ѧ����ʽ��
��2��0��t2ʱ����ڷ�Ӧ���������ԭ���ǣ� ��
��3��t2��tʱ����ڷ�Ӧ���ʼ�С��ԭ���ǣ� ��
��4��ͼ����Ӱ���֡��������ʾt1��t3ʱ���� ��
����Mn2+���ʵ���Ũ�ȵ����� B��Mn2+���ʵ���������
C��SO42-���ʵ���Ũ�� D��MnO4-���ʵ���Ũ�ȵļ�С
II.Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ����������ͼ�ס�����ʾ��ʵ�顣��ش�������⣺
��1�����Է���������ͼ�ɹ۲� �����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ�������������� ��
��2����������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ ��ʵ������Ҫ������������
��3������0.01mol MnO2��ĩ��60mL H2O2��Һ�У��ڱ�״���·ų����������ʱ��Ĺ�ϵ��ͼ��ʾ����ų�����������ΪV mL��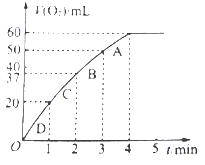
�ٷų�V/3 mL����ʱ����ʱ��Ϊ min��
�ڸ�H2O2��Һ��Ũ��Ϊ
��A��B��C��D���㷴Ӧ���ʿ�����˳��Ϊ > > >
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ��ݸ�и�����ѧ�ڵ��в������ۻ�ѧ�Ծ��������棩 ���ͣ�������
I����¯������ұ��������Ҫ��������������Ҫ��ӦΪ��
Fe2O3(s) + 3CO(g) 2Fe(s)+3CO2(g)
��H
2Fe(s)+3CO2(g)
��H
��1����֪����Fe2O3(s) + 3C(ʯī)=2Fe(s) + 3CO(g) ��H1
��C(ʯī��+ CO2(g) = 2CO(g) ��H2
���H___________________(�ú���H1 ����H2�Ĵ���ʽ��ʾ)��
��2����¯������Ӧ��ƽ�ⳣ������ʽK=____________________________��
��3����ij�¶�ʱ���÷�Ӧ��ƽ�ⳣ��K=64����2L�����ܱ����������У��ֱ��±���ʾ�������ʣ���Ӧ����һ��ʱ���ﵽƽ�⡣
|
|
Fe2O3 |
CO |
Fe |
CO2 |
|
��/mol |
1.0 |
1.0 |
1.0 |
1.0 |
|
��/mol |
1.0 |
1.5 |
1.0 |
1.0 |
�ټ�������CO��ƽ��ת����Ϊ_______________________��
������˵����ȷ����____________________(���ţ���
A��������ѹǿ�㶨����Ӧ�ﵽƽ��״̬
B���������������ܶȺ㶨����Ӧ�ﵽƽ��״̬
C����������CO��ƽ��ת���ʴ����ҵ�
D������Fe2O3�������CO��ת����
II������MgO�����������Ȼ�þ�ϳɡ�ijС���о��÷�Ӧ���¶�Ϊ378��398Kʱ�ķ�Ӧʱ�䡢��Ӧ����ȵ����ض�����ʵ�Ӱ�졣���������ʵ����Ʊ���
|
��� |
�¶�/K |
��Ӧʱ��/h |
��Ӧ�����ʵ������ |
ʵ��Ŀ�� |
|
�� |
378 |
4 |
3��1 |
ʵ��ں͢�̽��________ ______________________ ʵ��ں�__________̽�� ��Ӧʱ��Բ��ʵ�Ӱ�졣 |
|
�� |
378 |
4 |
4��1 |
|
|
�� |
378 |
3 |
_______ |
|
|
�� |
398 |
4 |
4��1 |
��ͼΪ�¶ȶ�����MgO���ʣ�����a��������(����b)��Ӱ�죬����ɳ��¶ȶ�����MgO�Ʊ���Ӱ����ɣ�д��һ������
___________________________________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���ӱ�ʡ10-11ѧ���һ��ѧ����ĩ���ԣ���ѧ����B�� ���ͣ�ʵ����
��ÿ��2�֣���22�֣�
I.ʵ����0.01mol/L��KMnO4��������Һ��0.1mol/L��H2C2O4��Һ�������Ϻ�Ӧ���ʦ�[mol/(L �� s)]�뷴Ӧʱ��t��s���Ĺ�ϵ��ͼ��ʾ���ش��������⣺

��1���÷�Ӧ�Ļ�ѧ����ʽ��
��2��0��t2ʱ����ڷ�Ӧ���������ԭ���ǣ� ��
��3��t2��tʱ����ڷ�Ӧ���ʼ�С��ԭ���ǣ� ��
��4��ͼ����Ӱ���֡��������ʾt1��t3ʱ���� ��
����Mn2+���ʵ���Ũ�ȵ����� B��Mn2+���ʵ���������
C��SO42-���ʵ���Ũ�� D��MnO4-���ʵ���Ũ�ȵļ�С
II. Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ����������ͼ�ס�����ʾ��ʵ�顣��ش�������⣺
��1�����Է���������ͼ�ɹ۲� �����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ�������������� ��
��2����������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ ��ʵ������Ҫ������������
��3������0.01mol MnO2��ĩ��60mL H2O2��Һ�У��ڱ�״���·ų����������ʱ��Ĺ�ϵ��ͼ��ʾ����ų�����������ΪV mL��
�ٷų�V/3 mL����ʱ����ʱ��Ϊ min��
�� ��H2O2��Һ��Ũ��Ϊ
��A��B��C��D���㷴Ӧ���ʿ�����˳��Ϊ > > >
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com