
ijѧ����0.200 0 mol��L��1�ı�N aOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�����Ϊ���¼�����
aOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�����Ϊ���¼�����
��������ˮϴ�Ӽ�ʽ�ζ��ܣ�������ע��NaOH��Һ����0���̶�������
�ڹ̶��õζ��ܲ�ʹ�ζ��ܼ������Һ��
�۵���Һ������0����0���̶�������ijһ�̶ȣ������¶���
����ȡ20.00 mL�� ��Һע��ྻ����ƿ�У�������3�η�̪��Һ
��Һע��ྻ����ƿ�У�������3�η�̪��Һ
���ñ�Һ�ζ����յ㣬���µζ���Һ�����
��ش�
(1)���ϲ����д������________________(����)���ô�������ᵼ�²ⶨ���(�ƫ����ƫС������Ӱ�족)________________��
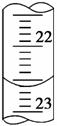
(2)�жϵζ��յ�������ǣ���ƿ����Һ��________ɫ��Ϊ________ɫ���Ұ�����ڲ���ɫ��
(3)��ͼ��ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ________ mL��
(4)�����������ݣ���������������Һ��Ũ��Ϊ________ mol��L��1��
| �ζ� ���� | ����Һ ���(mL) | ��NaOH��Һ������¼(mL) | |
| �ζ�ǰ���� | �ζ������ | ||
| ��һ�� | 20.00 | 0.40 | 20.40 |
| �ڶ��� | 2 | 4.0 | 24.00 |
| ������ | 20.00 | 2.00 | 24.10 |
������(1)��������ˮϴ�Ӽ�ʽ�ζ��ܺ�������ע��NaOH��Һ��Ӧ����NaOH��Һ��ϴ����������NaOH��Һ�����ƫ���²ⶨ�����Ũ��ƫ��(2)��̪�Լ���������Һ�г���ɫ������Һ��������ʱ���� Һ�ʷۺ�ɫ��(4)ȡǰ��������NaOH��Һ�����ƽ��ֵ(���������ϴ���ȥ)��Ȼ����빫ʽ���м��㣺c(��)V(��)��c(��)V(��)����c(��)��c(��)V(��)/V(��)��
Һ�ʷۺ�ɫ��(4)ȡǰ��������NaOH��Һ�����ƽ��ֵ(���������ϴ���ȥ)��Ȼ����빫ʽ���м��㣺c(��)V(��)��c(��)V(��)����c(��)��c(��)V(��)/V(��)��
�𰸡�(1)�١�ƫ��(2)�ޡ��ۺ졡(3)22.60��(4)0.200 0


 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ���ںϳ����в������з�Ӧ�ϳɼ״���
CO(g)��2H2(g)  CH3OH(g)����H��Q kJ��mol��1
CH3OH(g)����H��Q kJ��mol��1
(1)�±����������Ǹÿ��淴Ӧ�ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ��(K)
| �¶� | 250 �� | 300 �� | 350 �� |
| K | 2.041 | 0.270 | 0.012 |
���ɱ��������ж�Q________0(�����������������)���÷�Ӧ��________(��ϸߡ��ϵ͡�)�¶����������Է����С�
��ij�¶��£���2 mol CO��6 mol H2����2 L���ܱ������У���ַ�Ӧ�ﵽƽ����c(CO)��0.2 mol��L��1����ʱ��Ӧ���¶�Ϊ____________��CO��ת����Ϊ____________��
H2����2 L���ܱ������У���ַ�Ӧ�ﵽƽ����c(CO)��0.2 mol��L��1����ʱ��Ӧ���¶�Ϊ____________��CO��ת����Ϊ____________��
(2)����ͼ�л���ѹǿ��ͬ��ƽ��ʱ�״����������(��)���¶�(T)�仯����������(�������ϱ��p1��p2����p1��p2)��

(3)Ҫ���CO��ת��������߷�Ӧ���ʣ����Բ�ȡ�Ĵ�ʩ��________(����ĸ���)��
a�����¡�b�����������c������CO��Ũ�ȡ�d��ͨ��H2��ѹ��e��ͨ����������ѹ��f��������״�
(4)��֪һ�� �����£�CO��H2�ڴ���������������5 mol CH3OHʱ����
�����£�CO��H2�ڴ���������������5 mol CH3OHʱ���� ���ı仯Ϊ454 kJ���ڸ������£����ݻ���ͬ��3���ܱ������У����ռס��ҡ������ֲ�ͬ��Ͷ�Ϸ�ʽ���뷴Ӧ���÷�Ӧ�ﵽƽ��ʱ���й��������£�
���ı仯Ϊ454 kJ���ڸ������£����ݻ���ͬ��3���ܱ������У����ռס��ҡ������ֲ�ͬ��Ͷ�Ϸ�ʽ���뷴Ӧ���÷�Ӧ�ﵽƽ��ʱ���й��������£�
| ƽ��ʱ���� | ���� | �� | �� | �� |
| ��Ӧ��Ͷ���� | 1 mol CO 2 mol H2 | 1 mol CH3OH | 2 mol CH3OH | |
| CH3OH��Ũ��(mol��L��1) | c1 | c2 | c3 | |
| ��Ӧ���ջ�ų�������(kJ) | a | b | c | |
| ��ϵѹǿ(Pa) | p1 | p2 | p3 | |
| ��Ӧ��ת���� | ��1 | ��2 | ��3 |
����˵����ȷ����________(����ĸ���)��
A��2c1��c3�������������� B��a��b��90.8
C��2p2��p3 D����1����3��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ���εġ����硱����ˮ�к��и������࣬����70%Ϊ�Ȼ��ƣ���������Ȼ�þ������þ�ȡ�ij��ѧ��ȤС��Ϊ�˴Ӻ�ˮ�з�����Ȼ��ƣ����������ʵ�鷽����
��ˮ ��Һ
��Һ ��Һ
��Һ NaCl��Һ
NaCl��Һ NaCl
NaCl
(1 )��������Լ�AΪ________���������A��Ŀ����________________________��
)��������Լ�AΪ________���������A��Ŀ����________________________��
(2)��������Լ�BΪ________���������B��Ŀ����________________________��
(3)��������Լ�CΪ________����������C��________����Ŀ����________________________��
(4)�������ʵ�����Ϊ________���Ը���ͼʾ�ش�
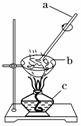
�ٰ�˳��д��ͼ�б�����������ƣ�_______________________________��
������a��������____________________����Ŀ����____________________��
�۵�����b�г���________ʱ����ֹͣ���ȡ�
(5)�û�ѧ��ȤС���÷���õ����Ȼ�������100 mL 1 mol/L��NaCl��Һ�����ƹ���������������ƽ��ȡ���ε�����Ϊ________g�����ڶ��ݵIJ��������Ĺ���������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ��������Mg��Zn��Al�����������ϡ���ᷴӦ����������2.8L������£���ԭ���������������ǣ� ��
A��2g B��4g C��8g D��10g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
374 �桢22.1 MPa���ϵij��ٽ�ˮ���к�ǿ���ܽ��л���������������н϶��H����OH�����ɴ˿�֪���ٽ�ˮ
A�������ԣ�pH����7
B�����ֳ��Ǽ����ܼ�������
C�������ԣ�pHС��7
D�����ֳ������ܼ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪NaHSO4��ˮ�еĵ��뷽��ʽΪ��NaHSO4===Na����H����SO ��ij�¶��£���pH��6������ˮ�м���NaHSO4���壬�����¶Ȳ��䣬�����Һ��pHΪ2�����ڸ���Һ�����������в���ȷ����
��ij�¶��£���pH��6������ˮ�м���NaHSO4���壬�����¶Ȳ��䣬�����Һ��pHΪ2�����ڸ���Һ�����������в���ȷ����
A�����¶��¼�������pH��12��NaOH��Һ��ʹ��Ӧ�����Һǡ��������
B��ˮ���������c(H��)��1��10��10 mol��L��1
C��c(H��)��c(OH��)��c(SO )
)
D�����¶ȸ���25 ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
25 ��ʱ�����ΪVa��pH��a��ijһԪǿ����Һ�����ΪVb��pH��b��ijһԪǿ����Һ���Ȼ�Ϻ���Һ��pH��7����֪b��6a��Va��Vb�������й�a��˵������ȷ����
A��a���ܵ���1 B��aһ������2
C��aһ��С��2 D��aһ������2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�ᴿ��������(������Ϊ���е���������)��ѡ�õij����Լ��ͷ��뷽������ȷ����(����)
| ���ᴿ������ | �����Լ� | ���뷽�� | |
| A. | NaBr��Һ(NaI) | ��ˮ��CCl4 | ��ȡ����Һ |
| B. | NH4Cl��Һ(FeCl3) | NaOH��Һ | ���� |
| C. | CO2(CO) | CuO��ĩ | ͨ�����ȵ�CuO��ĩ |
| D. | SiO2(Al2O3) | NaOH��Һ | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����a mol��L��1NaX��b mol��L��1NaY��������Һ������˵����ȷ����(������Һ��ϣ�����Ի��ʱ������仯)
A����a��b��pH(NaX)��pH(NaY)��������HX��HY
B����a��b��c(X��)��c(Y��)��c(HY)��������HX��HY
C����a��b��c(X��)��c(Y��)��������HX��HY
D����a��0.1 mol��L��1������Һ�������ϣ���c(X��)��c(HX)��0.1 mol��L��1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com