
����Ŀ��п������������Ԫ�أ�[Zn(NH3)4]CO3��������Եȷ��淢����Ҫ�����á�
��1��Zn2+��̬��������Ų�ʽΪ__��
��2��CO32-�Ŀռ乹��Ϊ__(����������)��[Zn(NH3)4]CO3��C��H��O��N����Ԫ�صĵ縺����С�����˳��Ϊ__��
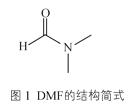
��3��ij��п����������ģ��̼����ø�Ĵ����ԣ���������к���DMF���ӡ�DMF���ӵĽṹ��ͼ1��ʾ��DMF������̼ԭ�ӹ�����ӻ�������__��1molDMF�����к�����������ĿΪ__��
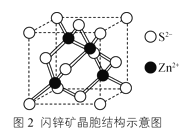
��4����п��ɿ�����Zn2+��S2-�����γɵ����������ṹ�������ɡ��侧���ṹʾ��ͼ��ͼ2��ʾ����Zn2+��������������Zn2+��__����
���𰸡�[Ar]3d10��1s22s22p63s23p63d10 ƽ���������� H<C<N<O sp2��sp3 11mol��11��6.02��1023 12
��������
��1��Zn�ǵ�30��ԭ�ӣ�Zn2+�Ļ�̬��������Ų�ʽΪ[Ar]3d10��1s22s22p63s23p63d10��
��2��CO32-�ļ۲���Ӷ���Ϊ![]() �����Կռ乹����ƽ�������Σ�ͬ����Ԫ�ش�����Ԫ�صĵ縺�����������е縺��C<N<O��H�ĵ縺����С���縺��˳��Ϊ��H<C<N<O��
�����Կռ乹����ƽ�������Σ�ͬ����Ԫ�ش�����Ԫ�صĵ縺�����������е縺��C<N<O��H�ĵ縺����С���縺��˳��Ϊ��H<C<N<O��
��3��DMF�У� ��1��λ�õ�C��˫������sp2�ӻ���2��3��λ�õ�C���ǵ�������sp3�ӻ�����������������˫����һ������һ������������DMF����11NA��������
��1��λ�õ�C��˫������sp2�ӻ���2��3��λ�õ�C���ǵ�������sp3�ӻ�����������������˫����һ������һ������������DMF����11NA��������
��4����ZnS�ľ����ṹ���Կ�����ZnS�������������ܶѻ���������Zn2+��������������Zn2+��12����


 Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ӹ���ķ�����Һ�к���2%��5%��NaNO2������һ�ֻ�����Ⱦ�������NH4Cl��Һ�������˷�����Һ��ʹ��������ת��Ϊ�����ʡ��÷�Ӧ���������У�
��һ����NaNO2+NH4Cl=NaCl+NH4NO2
�ڶ�����NH4NO2![]() N2��+2H2O
N2��+2H2O
���жԵڶ�����Ӧ����������ȷ����
��NH4NO2����������
��NH4NO2���ǻ�ԭ��
��NH4NO2�����˷ֽⷴӦ
��ֻ�е�Ԫ�صĻ��ϼ۷����˱仯
��NH4NO2�������������ǻ�ԭ��
A. �٢� B. �٢�
C. �ڢۢ� D. �ۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Һ�����ʵ���Ũ��Ϊ1mol��L-1����( )
A. ��20g NaOH�����ܽ���500mLˮ��
B. ��10g NaOH�����ܽ���ˮ�����250mL��Һ
C. ��1L 10 mol��L-1��Ũ������9Lˮ���
D. ����״����22.4L HCl��������1Lˮ�������Һ(��֪HCl��������ˮ,0��ʱ,1���ˮ���ܽ�500������Ȼ���)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Ҫ0.1mol��L��1NaOH��Һ450mL��0.5mol��L��1������Һ500mL��������������Һ����������ش��������⣺
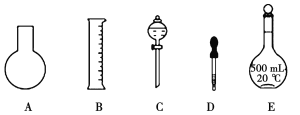
(1)��ͼ��ʾ��������������Һ�϶�����Ҫ����__________(�����)������������Һ�����õ��IJ���������__________(����������)��
(2)����0.1mol��L��1NaOH��Һ�����������£�
�ٰѳ����õ�NaOH`�������С�ձ��У�����������ˮ�ܽ⣻
�ڰ���������Һ��ȴ�����£���С��ת��һ���ݻ�������ƿ�У�
�ۼ���������ƿ�м�����ˮ��Һ���̶���1��2cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ��Һ����̶������У�
������������ˮϴ���ձ��Ͳ�����2��3�Σ�ÿ��ϴ�ӵ�Һ�嶼С��ת������ƿ��������ҡ�ȣ�
�ݽ�����ƿƿ�����������ҡ�ȡ�
�����������ȷ˳��Ϊ__________(�����)��
(3)���ݼ�����������ƽ��ȡNaOH������Ϊ__________g����ʵ����������������ȷ��������ƿ������ˮϴ�Ӻ�δ�����������ҺŨ��______________0.10mol��L��1(����������������������С��������ͬ)������δ����Һ��ȴ�Ͷ����ˣ���������ҺŨ��__________0.10mol��L��1��
(4)���ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84g��cm��3��Ũ��������Ϊ__________mL(����������һλС��)�����ʵ������10mL��15mL��20mL��50mL����Ͳ��Ӧѡ��__________mL����Ͳ��á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����13g Zn���������������У�Zn��ȫ��Ӧ������:
(1)13g Zn�����ʵ���___________
(2)�μӷ�Ӧ��HCl�����ʵ���___________
(3)����H2�����(��״��)___________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧУ��ѧѧϰС��Ϊ̽���������������ʣ�����ͼ��ʾװ�ý���ʵ�顣

��1��װ�ü���ʢ��Ũ���������A��������______����װ���з�����Ӧ�Ļ�ѧ����ʽΪ________________________��
(2) װ�ñ��е��Թ��ڷ�����Ӧ�����ӷ���ʽΪ��_______________________��
��3��Ϊ��̽��NO�Ļ�ԭ�ԣ�������װ�ö��ĵ�����C��ͨ��һ�����壬ͨ������������������________��
��4��ȡ��װ�ñ��е��Թ�D�������еμ�FeSO4��Һ����Һ��Ϊ________ɫ��Ϊ��֤����Ԫ���ڸ÷�Ӧ�еIJ������������Һ�еμ�KSCN��Һ����Һ��Ϊ________ɫ��
��5�����᳧���ô���ԭ��������β����CH4�ڴ������¿��Խ�NO2��ԭΪN2��
��֪��CH4(g)��2O2(g)===CO2(g)��2H2O(g)��H����889.6 kJ��mol��1��
N2(g)��2O2(g)===2NO2(g)��H����67.7 kJ��mol��1��
��CH4��ԭNO2����ˮ�����͵������Ȼ�ѧ����ʽ��_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ѡ���������ܴ���������ǣ� ��
(1)�����۷�Ӧ�ų�H2����ɫ��Һ��NO3-��Al3����Na����SO42-
(2)���д���NO3-����Һ��H����Fe2����Cl����SO42-
(3)������Һ��Fe3����Al3����NO3-��SO42-
(4)ʹpH��ֽ����ɫ����Һ��Cu2����NO3-��Fe3����SO42-
(5)���д���Fe3������Һ��Na����SCN����Cl����I��
(6)������![]() ��1��10��12����Һ��K����AlO2-��CO32-��Na��
��1��10��12����Һ��K����AlO2-��CO32-��Na��
(7)c(H��)��0.1 mol��L��1����Һ��Na����NH4+��SO42-��S2O32-
A.��3����6��B.��2����3����6��C.��6��D.��2����6����7��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��þһ���������һ���ܱ�ˮ�����һ���Դ�����أ�ԭ����ͼ��ʾ������˵���������

A. �ŵ�ʱ�����·������þ�缫������̼���ϵ缫
B. �ŵ�ʱ�������ĵ缫��ӦʽΪO2+ 4e��+ 2H2O =4OH
C. �����ϣ����·������2 mol����ʱ��������������58 g
D. ��ط�Ӧ����Mg(OH)2���������뻹ԭ���Ƴ�þ��ѭ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ҫ�Ե⻯����ʽ���ڡ���һ��ѧ����С���ú���Ϊԭ����ȡ�����ⵥ�ʣ����ǽ��������ճɻң���ˮ����һ��ʱ��(�õ⻯�����ܽ���ˮ��)���õ�����������Һ��Ȼ������ʵ��������ȡ���ʵ⣺
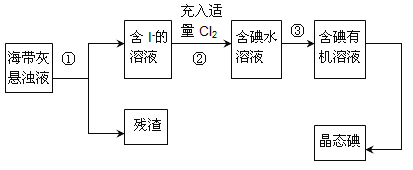
(1)ָ����ȡ��Ĺ������йص�ʵ��������ƣ���________����________��
(2)�����������õ��л��Լ�������________��
A���Ҵ� B�����Ȼ�̼ C������ D������
(3)��֪����������еõ���������ˮ��Һ����Cl-���Ӵ��ڣ�д���ò�������ӷ�Ӧ��___________________________________________��
(4)�Ӻ�����л���Һ����ȡ��ͻ����л���Һ������Ҫ�������۲���ͼ��ʾʵ��װ�ã�ָ������N�������ǣ�________________________��װ�����е������������Ը�������________________________����_______________________________��
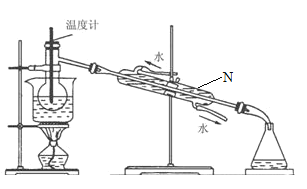
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com