
 ij������Ʒ�к��������Ȼ������ʣ�Ϊ�˲ⶨ����Ʒ���ȣ���ͬѧ�������ͼ��ʾװ�ú�ʵ�鷽����ʵ�鲽�����£�
ij������Ʒ�к��������Ȼ������ʣ�Ϊ�˲ⶨ����Ʒ���ȣ���ͬѧ�������ͼ��ʾװ�ú�ʵ�鷽����ʵ�鲽�����£�| ��� | �Ľ���� | ȱ��ʱ��Ӱ�� |
| �� | ||
| �� | ||
| �� |
| 106 |
| x |
| 44 |
| m2-m1 |
| 53 |
| 22 |
| 53 |
| 22n |
| 53 |
| 22n |
| ��� | �Ľ���� | ȱ��ʱ��Ӱ�� |
| �� | ͨ�벻��CO2�Ŀ�������N2��ʹ���ƿ�в�����CO2ȫ���ų� | ƫС |
| �� | ͨ�벻��CO2�Ŀ�������N2���ų���Ӧǰװ���еĿ��� | ƫ�� |
| �� |


 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
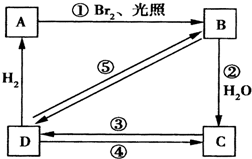 ��1���±�Ϊϩ��������巢���ӳɷ�Ӧ��������ʣ�����ϩΪ������
��1���±�Ϊϩ��������巢���ӳɷ�Ӧ��������ʣ�����ϩΪ������| ϩ����� | ������� |
| ��CH3��2C=CHCH3 | 10.4 |
| CH3CH=CH2 | 2.03 |
| CH2=CH2 | 1.00 |
| CH2=CHBr | 0.04 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ں�����Fe3+����Һ�У�NH4+��Na+��Cl-��SCN- |
| B����ǿ����Һ�У�Na+��K+��AlO2-��CO32- |
| C������SO42-���ڵ���Һ�У�Na+��Mg2+��Ca2+��I- |
| D����������Һ�У�K+��MnO4-��Fe2+��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ba��OH��2?8H2O��NH4Cl��Ӧ |
| B������ϡ���ᷴӦ |
| C�����ȵ�̿��CO2��Ӧ |
| D��������O2��ȼ�շ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
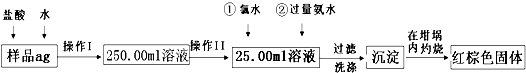
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
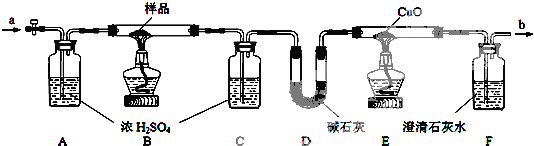
| ��Ʒ������ | Cװ������ | Dװ������ | Eװ�ü��� |
| 12.6g | 5.4g | 4.4g | 1.6g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com