
�״�����Ϊȼ�ϵ�ص�ԭ�ϡ���CH4��H2OΪԭ�ϣ�ͨ�����з�Ӧ���Ʊ��״���
I��CH4 ( g ) �� H2O ( g )��CO ( g ) �� 3H2 ( g )
II��CO ( g ) �� 2H2 ( g )��CH3OH ( g )
��1����Ӧ��I���ƵõĻ�������ˮú���ɷ���ͬ��Ҳ���γ�ȼ�ϵ�ء���ͼ����������ԭ�ϣ���������̼����Ϊ����ʵ�ȼ�ϵ�ع���ԭ��ʾ��ͼ����õ�صĸ�����Ӧʽ�ɱ�ʾΪ��
��
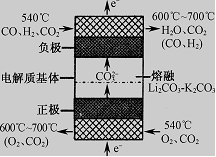
��2����ʹ�ü״�ʱ�����в�������ˮ�ʻ����һ������Ⱦ����һ�ֵ绯ѧ��������������Ⱦ����ԭ���ǣ�ͨ���Co2��������Co3����Ȼ����Co3������������ˮ�еļ״�������CO2��������ʵ��������ͼװ��ģ���������̣�
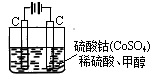
��д�������缫��Ӧʽ ��
��д����ȥ�״������ӷ���ʽ ��
�����㡿���⿼���˵�⡢ԭ���ԭ�������͵缫��Ӧ��д��������Ŀ�Ѷ��еȡ�
����������1�����ݷ�Ӧ�ı��ʿ�֪��CO��H2���������ڸ����μӷ�Ӧ������̼����Ϊ����ʣ����Ե缫��ӦΪCO��CO32����2e��=2CO2��H2��CO32����2e��=H2O��CO2��
��2����ͨ���Co2+������Co3+������������ʧ���ӷ���������Ӧ���缫��ӦΪCo2+-e-=Co3+��
�ʴ�Ϊ��Co2+-e-=Co3+������Co3+����������ˮ�еļ״�������CO2����������������ԭΪCo2+�����ԭ���غ������غ��֪����ԭ����H+����ƽ��д���ӷ���ʽΪ��6Co3++CH3OH+H2O=CO2��+6Co2++6H+��
���𰸡���8�֣�
��1��CO��CO32����2e��=2CO2��2�֣���H2��CO32����2e��=H2O��CO2��2�֣�����CO�� 3H2��4CO32����8e��=3H2O��5CO2��4�֣���
��2��Co2����e��=Co3�� ��2�֣� 6Co3����CH3OH��H2O=CO2��6Co2����6H����2�֣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ��ȤС���ͬѧ�ǰ��������ʵ�鷽���Ʊ������������壺����ȡ��������ˮ�ڽྻ���ձ��У��þƾ��Ƽ��������ڣ����ձ�����εμӱ��͵�FeCl3��Һ������У���Һ������ĺ��ɫ��
FeCl3��3H2O Fe(OH)3(����)��3HCl
Fe(OH)3(����)��3HCl
(1)�жϽ����Ʊ��Ƿ�ɹ��������ý����____________________��
(2)�����Ʊ��������������ʵ��ʱ����Щͬѧû�а�Ҫ����У����û�й۲쵽���壬����Ԥ����������ԭ��
�ټ�ͬѧû��ѡ�ñ����Ȼ�����Һ�����ǽ�ϡ�Ȼ�����Һ�����ˮ�У����û�й۲쵽________________����ԭ����______________________________________________________________��
����ͬѧ��ʵ����û��ʹ������ˮ������������ˮ�������________________��ԭ����__________________________________________
____________________________________________________��
�۱�ͬѧ���ˮ�еμӱ����Ȼ�����Һ��ʱ����ȣ������_______ _________��ԭ����_________________________________________
_________��ԭ����_________________________________________
_______________________________________________________��
(3)��ͬѧ��Ҫ���Ʊ���Fe(OH)3���壬����������Fe(OH)3��������μ�����ϡH2SO4��Һ�����������һϵ�б仯��
���ȳ��ֺ��ɫ������ԭ����___________________________________
________________________________________________________��
���������ܽ⣬�˷�Ӧ�����ӷ���ʽΪ_________________________
__________________________ ________________________________��
________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͭ������ϡ���ᣬ������������Һ����ͭ���������Ρ��ֽ�һ������ͭƬ���뵽100 mLϡ������������Ļ��Һ�У�ͭƬ��ȫ�ܽ�(�������ε�ˮ�⼰��Һ����ı仯)
(1)д��ͭ�ܽ����� �����Һ�����ӷ���ʽ_____________________________��
�����Һ�����ӷ���ʽ_____________________________��
(2)��ͭ��ȫ�ܽ�ʱ����Һ�е�Fe3����Cu2����H���������ӵ����ʵ���Ũ����ȣ��Ҳ����Һ��pH��1�����ܽ�ͭ��������________ g����Һ�е�c(SO )��________ mol/L��
)��________ mol/L��
(3)��������ͼ��ʾ��װ���з���(1)�еķ�Ӧ�����ж�ͼ�е�������������ѡ���ʵ����������缫��д���缫��Ӧʽ��������Ӧ�ı����С�
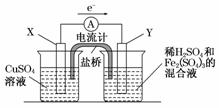
| ���������ж� | �缫���� | �缫��Ӧʽ | |
| X�� | |||
| Y�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڵ�3�����У��û��������������ǿ��Ԫ�ص�Ԫ�ط���Ϊ ����ѧ�������ȶ���Ԫ�ط����� ������������ˮ�����������ǿ�Ļ�����Ļ�ѧʽ�� ������������ˮ����ļ�����ǿ�Ļ�����Ļ�ѧʽ�� �������Ե���������Ļ�ѧʽ�� ���������������������ᡢ��������Һ�ֱ�Ӧ�����ӷ���ʽΪ �� ��ԭ�Ӱ뾶���Ľ���Ԫ�ص������� �����Ӱ뾶��С�����ӽṹʾ��ͼ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�缫��ÿͨ��96 500 C�ĵ����ͻ���1 mol���ӷ���ת�ơ���ȷ�������������ڶ��Ե缫���ԶƲ���ʽ�����Ľ�������������ȷ����������ͨ�����صĵ�����ʵ�ʲ����У������������ƣ���ͼ��ʾ������˵������ȷ����

A���������е�����Ӧ���Դ�������������������Ϸ����ĵ缫��Ӧ�ǣ�Ag����e��= Ag
B���������ǰ�������������仯���ý������ij�����Ϊ108.0 mg�����������ͨ�����صĵ���Ϊ96.5 C
C��ʵ���У�Ϊ�˱������ܽ�����п��ܲ����Ľ����������������������²������������缫��������һ���ռ���������û���ռ����������������ƫ�ߡ�
D����Ҫ�ⶨ��ⱥ��ʳ��ˮʱͨ���ĵ������ɽ������������е������������ص��������������������Դ�ĸ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�Ԫ�ؼ��仯�������ʵıȽ���ȷ����
A��ԭ�Ӱ뾶��F<O<Al B���ȶ��ԣ�H2O<PH3<NH3
C�����ԣ�H2CO3<H2SO4<H3PO4 D�����ԣ�LiOH<NaOH<Al(OH)3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йػ�ѧʵ���˵����������
A��Ϊ�˳�ȥNaCl��Һ�е�MgCl2���ɼ�����������Һ����Һ������
B����ij��Һ�м�����HCl�ữ��Ba(NO3)2��Һ��������ɫ�����������Һ�к���SO42-
C��ij������NaOHŨ��Һ���ȣ�����ʹʪ���ɫʯ����ֽ���������壬�������к���NH4+
D������ʵ���ʣ��Ľ�����Ӧ�Ż��Լ�ƿ��������ȫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��þ���Ͻ������Ĵ������š�����������Ʒ���ɡ����ۡ����á���������Щ�ص��ص�þ���Ͻ�������� �� ��
A���������� B�������Ժ� C���ܶ�С D��ǿ�ȸ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ��þ���Ͻ�Ͷ�뵽1 mol��L��1 HCl��Һ����Ͻ���ȫ�ܽ������Һ�����1 mol��L��1NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ����仯�Ĺ�ϵ����ͼA��ʾ������˵���в���ȷ����
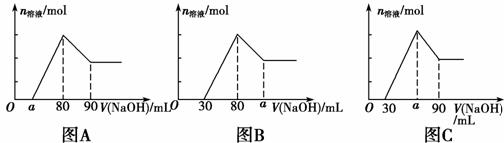
A��������ϵͼ��ΪBͼʱ����a��ȡֵ��ΧΪ80��a��90
B.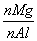 �����ֵΪ2.5
�����ֵΪ2.5
C��������ϵͼ��ΪCͼʱ����a��ȡֵ��ΧΪ75��a��90
D��a��ȡֵ��ΧΪ0��a��50
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com