
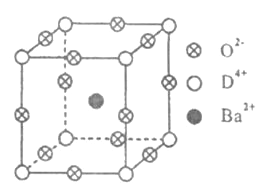
����Ŀ����֪A��B��C��D����Ԫ�ص�ԭ������֮�͵���36.A�ĵ�������������壻B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�С����������֮�ƣ��䵥�ʺͻ������й㷺����;��D4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ.��ҵ������DO2��̼�ᱵ������״̬����ȡ�������(�ɿ���һ�ֺ�������).��������������ġ�ѹ�����ܡ���Ӧ���ڳ������ķ���װ��.��X���߷��������������ľ����ṹΪ������(����ͼ��ʾ)������Ba2+ռ������λ�ã�O2-ռ������λ�ã�D4+ռ�ݶ���λ��.
��ش��������⣺
��1��A��B��C����Ԫ�صĵ縺���ɴ�С��˳����__________________(��Ԫ�ط���).
��2��BA4���ӵĿռ乹����______________��Bԭ�ӹ�����ӻ�����Ϊ_____.
��3��C����̬�⻯��ĵ���ʽΪ____����е����ͬ��������Ԫ���⻯��ķе㣬��Ҫԭ����____________________.
��4��D�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ____________________.
��5�����Ʊ�������Ļ�ѧ����ʽΪ____________________.
���ڼ����У�����D4+��������������ģ�Ba2+����������Ķ��㣬��O2-�����������__________.
���ڼ����У�D4+������λ��Ϊ__________.
����֪�����Ħ������ΪM g/mol���侧���߳�Ϊ4.03��10-10m���������ܶ�Ϊ__________________g/cm3(Ҫ���г���ʽ�������ӵ�������NA��ʾ).
���𰸡�N��C��H�� ���������� sp3 ![]() ��Ϊ����Ӽ���������������е����ͬ��������Ԫ���⻯��ķе� 1s22s22p63s23p63d24s2 BaCO3+TiO2=BaTiO3+CO2�� ���� 6
��Ϊ����Ӽ���������������е����ͬ��������Ԫ���⻯��ķе� 1s22s22p63s23p63d24s2 BaCO3+TiO2=BaTiO3+CO2�� ���� 6 ![]()
��������
A�ĵ�������������壬��AΪH��B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ���B�ĵ����Ų�ʽΪ1s22s22p2����BΪC��D�С����������֮�ƣ��䵥�ʺͻ������й㷺����;��D4�����Ӻ��ԭ�ӵĺ�������Ų���ͬ����D��ԭ������Ϊ22��DΪTi��ԭ�������Ժ˵���36����CΪN��Ȼ����з�����
A�ĵ�������������壬��AΪH��B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ���B�ĵ����Ų�ʽΪ1s22s22p2����BΪC��D�С����������֮�ƣ��䵥�ʺͻ������й㷺����;��D4�����Ӻ��ԭ�ӵĺ�������Ų���ͬ����D��ԭ������Ϊ22��DΪTi��ԭ�������Ժ˵���36����CΪN��
��1��ͬ���ڴ������ҵ縺�������縺�Դ�С˳����N>C>H��
��2��BA4ΪCH4���ռ乹��Ϊ�������壬����ԭ��C��4���Ҽ����µ��ӣ��۲���Ӷ���Ϊ4�����ӻ�����Ϊsp3��
��3��C����̬�⻯��ΪNH3�������ʽΪ![]() ���������Ӽ�������������ͬ�����⻯�ﲻ�����Ӽ��������˰����е����ͬ��������Ԫ���⻯��ķе㣻
���������Ӽ�������������ͬ�����⻯�ﲻ�����Ӽ��������˰����е����ͬ��������Ԫ���⻯��ķе㣻
��4��DΪTi�����̬ԭ�Ӻ�������Ų�ʽΪ 1s22s22p63s23p63d24s2��
��5���ٸ��ݻ�����ľ����ṹ��Ba2��λ���ڲ���Ba2������Ϊ1��Ti4��λ�ڶ��㣬����Ϊ8��1/8=1��O2��λ�����ϣ�����Ϊ12��1/4=3���Ļ�ѧʽΪBaTiO3����˻�ѧ��Ӧ����ʽΪBaCO3��TiO2=BaTiO3��CO2����
������Ti4����������������ģ�Ba2������������Ķ��㣬���ݾ����Ľṹ���Ƴ�O2��λ�����ģ�
��Ti4��������λ����ָ���Ǿ���Ti4�������O2���ĸ��������ݾ����Ľṹ���Ƴ�Ti4����������λ��Ϊ6��
�ܾ���������Ϊ![]() g�����������Ϊ(4.03��10��10��102)3cm3�������ܶȵĶ��壬�ó�������ܶ�Ϊ
g�����������Ϊ(4.03��10��10��102)3cm3�������ܶȵĶ��壬�ó�������ܶ�Ϊ ![]() g/cm3��
g/cm3��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��������ӵ�ص��ܷ�ӦʽΪ2Li+FeS=Fe+Li2S��LiPF6��SO(CH3)2Ϊ����ʣ��øõ��Ϊ��Դ��⺬�����Է�ˮ���õ�����Ni��ʵ��װ����ͼ��ʾ������˵������ȷ����

A. �缫YΪLi
B. �������У�b��NaCl��Һ�����ʵ���Ũ�Ƚ����ϼ�С
C. X����ӦʽΪFeS+2Li++2e-=Fe+Li2S
D. ����ͼ��������Ĥȥ������a��b���Һϲ������ⷴӦ�ܷ���ʽ�����ı�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������8�֣����в���Ԫ�ص�������ԭ��(�����)�ṹ���±���
Ԫ�ر�� | Ԫ��������ԭ��(�����)�ṹ |
T | �����������Ǵ�����������3�� |
X | �����µ���Ϊ˫ԭ�ӷ��ӣ������к���3�Թ��õ��Ӷ� |
Y | M���K����1������ |
Z | ��������Ԫ�صļ������а뾶��С |
(1)д��Ԫ��T��ԭ�ӽṹʾ��ͼ ��
(2)Ԫ��Y��Ԫ��Z��ȣ������Խ�ǿ����________(��Ԫ�ط��ű�ʾ)�����б�������֤����һ��ʵ����________(�����)��
a��Y���ʵ��۵��Z���ʵ�
b��Y�Ļ��ϼ۱�Z��
c��Y������ˮ��Ӧ��Z���ʾ���
d��Y����������ˮ����ļ��Ա�Zǿ
(3)T��X��Y��Z��������Ԫ�����γɼ������Ӽ����зǼ��Թ��ۼ��Ļ����д���û�����ĵ���ʽ��_____ _________��
(4)Ԫ��T����Ԫ�ؿ��γ�һ��18���ӵĻ�����Q��Ԫ��X����Ԫ��Ҳ�ܰ�ԭ�Ӹ�����Ϊ1��2�γ�һ��18���ӻ�����W��Q��W����������ԭ��Ӧ������X���ʺ�T����һ���⻯�д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�л���X�Ľṹ��ʽ��ͼ��ʾ�������й�˵������ȷ����
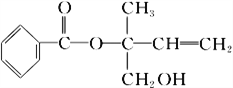
A. X�����к������ֹ�����
B. �������Ը��������Һ���𱽺�X
C. X��һ���������ܷ����ӳɡ��Ӿۡ�ȡ���������ȷ�Ӧ
D. �ڴ����������£�1 mol X�������5 mol H2�ӳ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ij������ϩ���Ļ������9.00g���û��������ܶ�Ϊ��ͬ״����������11.25�������������ͨ��������ˮ����ˮ��������4.20g����ԭ�����������Ϊ( )
A. ���� ����ϩB. ���� ����ϩC. ���� ����ϩD. ���� ����ϩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�������ķ���ʽΪC16H22O5��1mol������ȫˮ��ɵõ�1mol�����2mol�Ҵ���������ķ���ʽΪ
A.C12H6O5B.C12H18O5C.C12H12O4D.C12H14O5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ����ʾװ��ͨ��10 min����ֱ����Դ�����ӳ�ͼ����ʾװ�ã��ɹ۲쵽U�ι�������缫���������˰�ɫ��״���ʣ�U�ι��Ҳ�Һ������������˵����ȷ����( )

A. ͬ��ͬѹ�£�װ�â���ʯī�缫�Ϸ��õ�����������缫�Ϸ��õ��������
B. װ�â������缫�ĵ缫��ӦʽΪFe��2e����2OH��===Fe(OH)2��
C. װ�â���ʯī�缫�ĵ缫��ӦʽΪ2H����2e��===H2��
D. װ�â�ͨ��10 min�����缫������Һ��pH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D�������ʾ�Ϊ����������ɵĿ����Ի����������������ʵ����ӣ����Ӳ����ظ���ϣ��У�
������ | Na+��Al3+��Ba2+��NH4+ |
������ | Cl����OH����CO32����SO42�� |
�ֱ�ȡ�������ʽ���ʵ�飬ʵ����������B��Һ�ֱ���C��D��ϣ����а�ɫ������������A��Һ��ε���C��Һ�У��г������ɣ������μ�A��Һʱ����������ֱ����ȫ��ʧ��A��D���ֹ��������������ɣ���������ʹʪ��ĺ�ɫʯ����Һ��������ʯī�缫���B��Һ���������ϲ���һ���д̼�����ζ������
�ش��������⣺
��1��A�����������Ӻ�C���������ӵİ뾶��С____��______�������ӷ��ţ���B��������������________
��2��C��Һ��___�ԣ�����ԡ����ԡ�������ԭ����__________________
�������ӷ���ʽ���ͣ���D�Ļ�ѧʽ��____________
��3����PtΪ�缫���1L0.1mol/LB��ˮ��Һ������·��ͨ��0.1mol����ʱ��
��Һ��pHΪ_______�����������Һ������䣩�������ĵ缫��ӦʽΪ��_____
��4����������������������ͨ��A��Һ����ǡ����ȫ��Ӧʱ������Һ�и�����
Ũ���ɴ�С������˳��Ϊ__________________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com