
����Ŀ��ij��ѧС���Ա�����Ϊԭ����ȡ������������й����ʵķе����Է����������±���
���� | �״� | ������ | ��������� |
�е�/�� | 64.7 | 249 | 199.6 |
��Է������� | 32 | 122 | 136 |
�����ϳɱ���������ֲ�Ʒ
����ƿ�м���12.2g�������20mL�״�(�ܶ�Լ0.79g/mL)����С�ļ���3mLŨ���ᣬ���Ⱥ�Ͷ�뼸�����Ƭ��С�ļ���ʹ��Ӧ��ȫ���ñ���������ֲ�Ʒ
��1�����Һ��ʱ������Ũ�����������_____________________��Ũ�����������_____________������Ӧ����ˮ�����м���ͬλ��18O��д���ܱ�ʾ��Ӧǰ��18Oλ�õĻ�ѧ����ʽ____________��
��2���������һ��ʱ��������Ǽ����Ƭ��Ӧ�ò�ȡ����ȷ������____________��
��3���ס��ҡ�����λͬѧ�ֱ��������ͼ1����ʵ������ȡ�����������װ��(�г������ͼ�������������ȥ)�������л�����ص㣬��ò���װ��________(��ס������ҡ���������)�� 
�����ֲ�Ʒ�ľ���
��4������������ֲ�Ʒ���������������״������ᡢ�������ˮ�ȣ�������ͼ2����ͼ���о��ƣ���� ������ͼ����ǡ���������������ƣ�����IΪ_______��������Ϊ _______��
��5��ͨ�����㣬����������IJ���Ϊ____________��
���𰸡���1��Ũ�����ܶȽϴ����뱽���ᡢ�״���Ϸų�����������������ˮ�� C6H5CO18OH+CH3OH![]() C6H5COOCH3+H218O
C6H5COOCH3+H218O
��2����ȴ��
��3����
��4����Һ ����
��5��65%
��������
�����������1��Ũ�����ܶȽϴ����뱽���ᡢ�״���Ϸų������������ʻ����ҺʱӦ������Ũ���Ũ������������Ӧ�����˴������ã�������ӦΪ���淴Ӧ��Ũ�������շ�Ӧ���ɵ�ˮ�����Դٽ��������ɣ�Ũ����������ˮ�������ã���������״���Ũ���������µ�������ӦΪ����������ȥ�ǻ����ݴ���ȥ�ǻ��е���ԭ�ӣ����߷�Ӧ���ɱ��������������ˮ��18Oԭ�����Ա����ᣬ��Ӧ�Ļ�ѧ����ʽΪ��C6H5CO18OH+CH3OH![]() C6H5COOCH3+H218O��
C6H5COOCH3+H218O��
��2���������һ��ʱ��������Ǽ����Ƭ��Ӧ�ò�ȡ����ȷ��������ȴ����룬��ֹҺ�屩�У�
��3����װ��ͼ��֪����ͼ��Բ����ƿ���������ܣ�����������ã���ͼ�ͱ�ͼ��û�У��������з�Ӧ��״��е�ͣ������ᡢ����������ķе�Զ���ڼ״��������ü�ͼ����ͼ���״��ض�������ӷ��������ںϳɷ�Ӧ������Ӧ�������������ټ״��Ļӷ�����߲��ʣ��ʴ�Ϊ�ң�
��4�����������������ˮ��������������������ܵ�Һ�壬ͨ�����÷�Һ������ɣ��״��ͱ����������ܽ⣬���߷е㲻ͬ������ͨ������������룻
��5��12.2g����������ʵ���Ϊ��![]() =0.1mol��20mL�״�(�ܶ�Լ0.79g/mL)�����ʵ���Ϊ��
=0.1mol��20mL�״�(�ܶ�Լ0.79g/mL)�����ʵ���Ϊ��![]() =0.49mol��0.1mol��
=0.49mol��0.1mol��
�����������ɱ�������������ʵ���Ϊ��0.1mol������Ϊ��136g/mol��0.1mol=13.6g����������IJ���Ϊ��![]() ��100%=65%��
��100%=65%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ԫ����������Ԫ�����ڱ��е�λ�����£��ش��й����⣺
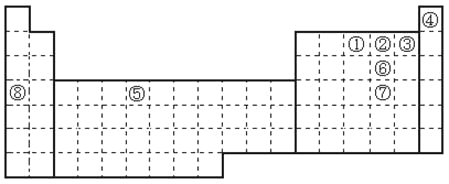
��1��д����������Ԫ���е�һ����������Ԫ�ط���___________��
��2��д���ڢޢ�����Ԫ����̬�⻯ѧ�ķе�˳��___________���������ʽ˳��
��3���뻭��[Cu��NH3��4]2+���ӽṹʾ��ͼ___________��
��4���뻭��������Χ�����Ų�ͼ___________��
��5��SeO3�Ŀռ乹��Ϊ___________��
��6��SeO32�����ӵ�VSEPR����Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ԫ����(HnX)��Һ��c(H��)���Ƚ���������ǿ��ʱ��ͨ��ֻ���ǵ�һ�����롣�ش����й��ڶ�Ԫ����HnX�����⡣
��1����ҪʹHnX��Һ��c(H��)/c(HnX)�����Բ�ȡ�Ĵ�ʩ��__________��
A�������¶� B����������̬HnX C��������NaOH��Һ D����ˮ
��2�������ӷ���ʽ����NanX�ʼ��Ե�ԭ��______________________________��
��3����HnXΪH2C2O4����ij�¶��£�H2C2O4��K1=5��10��2��K2=5��10��5������¶��£�0.2mol/L H2C2O4��Һ��c��H����ԼΪ__________mol/L������ȷ���㣬�Ҽ�֪![]() ��
��
��4����֪KHC2O4��Һ�����ԡ�
��KHC2O4��Һ�У�������Ũ���ɴ�С��˳����____________________��
����KHC2O4��Һ�У�������Ũ�ȹ�ϵ��ȷ����__________��
A��c(C2O42��)��c(H2C2O4)
B��c(OH��)=c(H��)��c(HC2O4��)��2c(H2C2O4)
C��c(K��)��c(H��)=c(OH������c(HC2O42��)��2c(C2O42��)
D��c(K��)=c(C2O42��)��c(HC2O4��)��c(H2C2O4)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ԭ���������������X��Y��Z��G��Q��R��T����Ԫ�أ��˵������С��36����֪X��Y��Z��Ԫ�صĻ�̬ԭ��2p�ܼ����е����ӣ������Ӹ����ֱ���2��3��2��G��Tԭ���������18��TԪ�������ڱ���ds���ĵ�һ��Ԫ�أ�Qԭ��s�ܼ���p�ܼ���������ȣ�R������������ּ���������Ӳ�Ʒ�ĺ��IJ��ϡ�
��1��Yԭ�Ӻ����________�ֲ�ͬ�˶�״̬�ĵ��ӣ�TԪ�ػ�̬ԭ����________�ֲ�ͬ�ܼ��ĵ��ӡ�________����״��ͬ��ԭ�ӹ����
��2��X��Y��Z�ĵ縺���ɴ�С��˳��Ϊ________��G��Q��R��һ��������С�����˳��Ϊ________(��Ԫ�ط��ű�ʾ)��
��3��YԪ�ػ�̬ԭ�ӵĵ����Ų�ͼ________��TԪ�ػ�̬ԭ�ӵļ۵����Ų�ʽ________��
��4��XZ2�ĵ���ʽΪ________���õ��뷽��ʽ��ʾY������⻯���ˮ��Һ�ʼ��Ե�ԭ��________��
��5��+1����̬��������ʧȥһ�������γ�+2����̬��̬����������Ҫ��������Ϊ�ڶ�������I2�����λ���I3��I4��I5���������Ʋ�GԪ�صĵ�����ͻ��Ӧ�����ڵ�________�����ܡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������(Na2S2O3)�����������ƺ����ͨ�����Ϸ�Ӧ�Ƶá���֪��Na2S2O3��������Һ�в����ȶ����ڡ�
��1��ij�о�С��������Ʊ�Na2S2O3��5H2O��װ�úͲ��ֲ���������ͼ��ʾ��
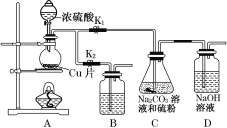
��.��K1���ر�K2����Բ����ƿ�м�������Ũ���ᣬ���ȡ�
��.C�еĻ��Һ��������������Ӧһ��ʱ�����۵������١���C����Һ��pH�ӽ�7ʱ��ֹͣC�еķ�Ӧ��
��.����C�еĻ��Һ��
��.����Һ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ���ɣ��õ���Ʒ��
�ٲ�����У�Բ����ƿ�ڷ�����Ӧ�Ļ�ѧ����ʽ��_________________��
�ڲ�����У�����C����Һ��pH�ӽ�7ʱ��ֹͣC�еķ�Ӧ����ԭ����____________����ֹͣC�еķ�Ӧ���IJ�����____________________________________��
��װ��B��ʢ�ŵ��Լ���________��Һ(�ѧʽ)����������___________________��
��2�����ݷ�Ӧ2S2O��I2��S4O��2I��������I2�ı���Һ�ⶨ��Ʒ�Ĵ��ȡ�ȡ5.5 g��Ʒ�����Ƴ�100 mL��Һ��ȡ10 mL��Һ���Ե�����ҺΪָʾ������Ũ��Ϊ0.050 mol��L��1�ı���Һ���еζ���������ݼ�¼�����ʾ��
��� | 1 | 2 | 3 | 4 |
��Һ�����/mL | 10.00 | 10.00 | 10.00 | 10.00 |
����I2����Һ�����/mL | 19.99 | 19.98 | 17.13 | 20.03 |
���жϴﵽ�ζ��յ��������__________________________________________��
��Na2S2O3��5H2O��ʽ��M=248���ڲ�Ʒ�е�����������(����������1λС��)________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijС����������װ����ȡ��̽�����������ʡ�
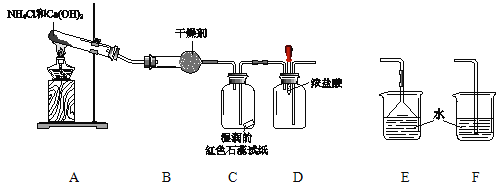
��1��A�з�Ӧ�Ļ�ѧ����ʽ�� ��
��2��B�еĸ������ ��
��3��C������� ��
��4��ʵ�����һ��ʱ���ѹDװ���еĽ�ͷ�ιܣ�����1��2��Ũ���ᣬ�ɹ۲쵽�������� ��
��5��Ϊ��ֹ�����������ݣ���Ҫ������װ�õ�ĩ������һ��β������װ�ã�Ӧѡ�õ�װ���� ���E����F������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪п���ܽ���NaOH��Һ�У�����H2��ijͬѧ�ݴ�����˲ⶨ��п��Ƥп�Ʋ��ȵ�ʵ�鷽�������������ΪS������Ϊm1�Ķ�п��Ƥ��ʯī�õ�������������6 mol��L-1 NaOH��Һ�У���ʯī���ϲ��������ݲ���ʱ��ȡ����Ƭ����ˮ��ϴ����ɺ������������Ϊm2 ������˵����ȷ����
A����п�Ʋ���Ϊh��п���ܶ�Ϊ�ѣ���![]()
B��п�缫�ĵ缫��ӦʽΪ2H2O+2e-==H2��+2OH-
C��п��ʯī�ڼ���Һ���γ�ԭ��أ����·�е�����п����ʯī
D����ʯī�ϲ���������ʱ������ȡ����Ƭ����ϴ���þƾ��Ƽ��Ⱥ�ɣ���Ƭ���ܲ��ֱ�����������ʵ����ƫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����HI(g)�����ܱ������У�ij�¶��·������б仯��2HI(g) ![]() H2(g)+I2(g)��H��0
H2(g)+I2(g)��H��0
��1���÷�Ӧƽ�ⳣ���ı���ʽΪK=______________����H2(g)+I2(g) ![]() 2HI(g)ƽ�ⳣ���ı���ʽΪK1=_____________(��K��ʾ)��
2HI(g)ƽ�ⳣ���ı���ʽΪK1=_____________(��K��ʾ)��
��2������Ӧ�ﵽƽ��ʱc(I2)=0.5mol/L��c(HI)=4mol/L����c(H2)Ϊ________��HI�ķֽ���Ϊ________��
��3�����жϸ÷�Ӧ�ﵽƽ��״̬��������________
A��������ѹǿ����
B�����������c(HI)����
C��c(I2)=c(H2)
D��v(HI)��=v(H2)��
��4�����÷�Ӧ800��ʱ�ﵽƽ��״̬����ƽ�ⳣ��Ϊ1.0��ijʱ�̣���������ڸ����ʵ�Ũ���ֱ�Ϊc(HI)=2.0mol/L��c(I2)=1.0mol/L��c(H2)=1.0mol/L�����ʱ�̣���Ӧ��_________(������������������������ͬ)���У��������¶ȣ���Ӧ��_________���С�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������-66������-66��������������������������˿���֯�������������·�����ϳɡ�D�ĺ˴Ź��������������塣
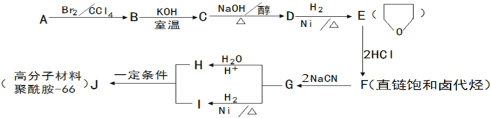
��֪��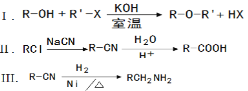
��1��1molA��1molH2��һ��������ǡ�÷�Ӧ�����ɱ���һԪ��X��X��̼Ԫ�ص���������ԼΪ65%����X�ķ���ʽΪ ��A���������������ŵ������� ��
��2��B��C�ķ�Ӧ������_________________��
��3��C��D�Ļ�ѧ����ʽΪ_______________________________________��
��4��F���������Ƶ��Ҵ���Һ���ȣ��õ��л�����Y
��Y��������_____________________��
��Y��һ�������¿��Է����Ӿ۷�Ӧ�õ���˳ʽ�ṹΪ����˳ʽ�ۺ����˳ʽ�ۺ���Ľṹ��ʽΪ_____________________________��
��5��H��I��Ӧ����J��������-66���Ļ�ѧ��Ӧ����ʽΪ_________________________��
Ϊ����F�еĹ����ţ������Լ�����NaOHˮ��Һ�� ��
��6��ͬʱ��������������H��ͬ���칹���ж��֡�д������һ��ͬ���칹��Ľṹ��ʽ(�˴Ź��������������壬�����Ϊ4:4:2)________________________��
��ֻ��һ�ֹ����� ��1mol������������������Һ��Ӧ������4molAg
��7����֪�� ![]()
![]()
д���Ա��Ӻ�HCHOΪԭ���Ʊ�![]() �ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com