
| 5.3 |
| 9.5 |
| 5.3 |
| 9.5 |


 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
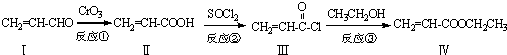
 Ҳ���뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ
Ҳ���뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
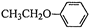 �ͣ�CH3CO��2OΪԭ���Ʊ�ҩ���м���
�ͣ�CH3CO��2OΪԭ���Ʊ�ҩ���м���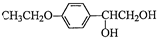 �ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�H2C=CH2
�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�H2C=CH2| HBr |
| NaOH��Һ |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

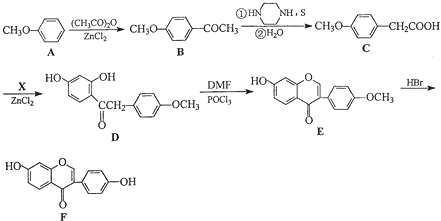
 �������ڷ�����д���ϳ�·�����̣����Լ���ѡ����ע���ϳ�·�����̵���д��ʽ��������ʾ����
�������ڷ�����д���ϳ�·�����̣����Լ���ѡ����ע���ϳ�·�����̵���д��ʽ��������ʾ����| Cl2 |
| ���� |
| NaOH/H2O |
| �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
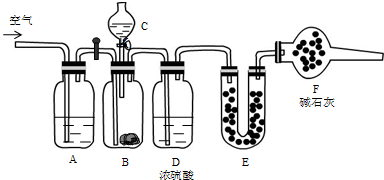
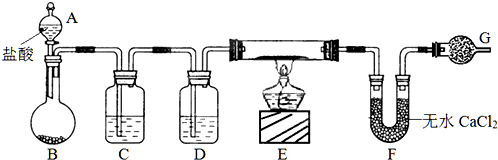
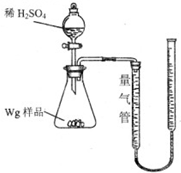 ����ͼ��ʾװ�òⶨFe��Fe2O3�Ļ�����е������������������гֲ�������ȥ������ȡWg��Ʒ������ƿ�У�ͨ����Һ©����������ϡ����ʹ��Ʒ��ȫ�ܽ⣮ʵ��ǰ��������ʼ����ΪamL��ʵ��������ܵ����ն���ΪbmL��
����ͼ��ʾװ�òⶨFe��Fe2O3�Ļ�����е������������������гֲ�������ȥ������ȡWg��Ʒ������ƿ�У�ͨ����Һ©����������ϡ����ʹ��Ʒ��ȫ�ܽ⣮ʵ��ǰ��������ʼ����ΪamL��ʵ��������ܵ����ն���ΪbmL��| ��� | ��Һ�п��ܴ��� �Ľ������� | ѡ�������������Լ�����֤�� ���裨����ĸ�� |
| �� | ||
| �� | ||
| �� |
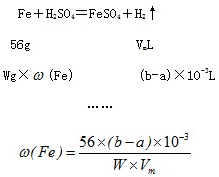
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Һ����ȡ������ |
| B��������ȡ����Һ |
| C����Һ��������ȡ |
| D����ȡ������Һ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com