
°ĺŐ‚ńŅ°ŅÓ—”ŽŐķ «ļ‹÷ō“™ĶńĹū Ű°£“—≥…ő™ĽĮĻ§…ķ≤ķ÷–÷ō“™Ķń≤ńŃŌ°£ĽōīūŌ¬Ń–ő Ő‚£ļ
£®1£©ĽýŐ¨Ó—‘≠◊”ĶńľŘĶÁ◊”ŇŇ≤ľ Ĺő™__________________£¨Ĺū ŰÓ—ĺßįŻ»ÁŌ¬◊ůÕľňý ĺ£¨ĺßįŻ≤ő żő™a=b= 295.08pm£¨c=468.55pm£¨¶Ń=¶¬=90%£¨y= 120%°£Ĺū ŰÓ—ő™______________∂—Ľż(ŐÓ∂—Ľż∑Ĺ Ĺ)°£
£®2£©”√»Ř»ŕĶń√ĺ‘ŕŽ≤∆Ý÷–ĽĻ‘≠TiCl4Ņ…Ķ√ĶĹ∂ŗŅ’Ķńļ£√ŗÓ—°£“—÷™TiCl4‘ŕÕ®≥£«ťŅŲŌ¬ «őř…ę“ļŐŚ£¨»ŘĶ„ő™-23°ś£¨∑–Ķ„ő™136°ś£¨Ņ…÷™TiCl4ő™____________ĺß ŐŚ°£
£®3£©Õ®ĻżX-…šŌŖŐĹ√ųKCl°ĘCaO°ĘTiNĺßŐŚ”ŽNaClĺßŐŚĹŠĻĻŌŗň∆£¨«“÷™ŃĹ÷÷ņŽ◊”ĺßŐŚĶńĺßłŮń‹ żĺ›»ÁŌ¬£ļ
ņŽ◊”ĺßŐŚ | KCl | CaO |
ĺßłŮń‹(kJ/mol) | 715 | 3401 |
Ĺ‚ ÕKClĺßłŮń‹–°”ŕCaOĶń‘≠“Ú£ļ_______________°£
Ó—Ņ…”ŽC°ĘN°ĘOĶ»‘™ňō–ő≥…∂Ģ‘™ĽĮļŌőÔ°£C°ĘN°ĘO‘™ňōĶńĶÁłļ–‘”…īůĶĹ–°Ķńň≥–Ú «________°£
(4)ł∆Ó—ŅůĺßŐŚĶńĹŠĻĻ»ÁŌ¬”“Õľňý ĺ°£ĺßŐŚĶńĽĮ—ß Ĺő™_________________°£
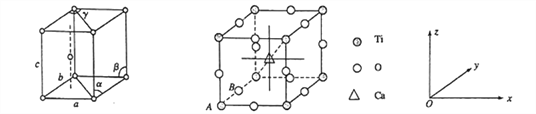
ĺßįŻ÷–Ķń‘≠◊”Ņ…”√x°Ęy°Ęz◊ť≥…Ķń»ż ż◊ťņīĪŪīÔňŁ‘ŕĺßįŻ÷–ĶńőĽ÷√£¨≥∆ő™‘≠◊”◊ÝĪÍ°£“—÷™‘≠◊”◊ÝĪÍő™A(0£¨0£¨0)£ĽB(0£¨1/2£¨0)£Ľ‘ÚCa ņŽ◊”Ķń‘≠◊”◊ÝĪÍő™______________°£
£®5£©Fe”–¶ń°Ę¶√°Ę¶Ń»ż÷÷Õ¨ňō“ž–őŐŚ£¨∆šĺßįŻĹŠĻĻ»ÁŌ¬Õľňý ĺ£ļ
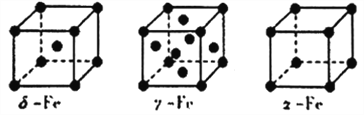
ĘŔ¶ń°Ę¶ŃŃĹ÷÷ĺßŐŚĺßįŻ÷–Őķ‘≠◊”ĶńŇšőĽ ż÷ģĪ»ő™_______________________°£
Ęŕ»ŰFe‘≠◊”įŽĺ∂ő™rpm£¨NAĪŪ ĺįĘ∑Łľ”Ķ¬¬ř≥£ żĶń÷Ķ£¨‘Ú¶ń-FeĶ•÷ Ķń√‹∂»ő™________g/cm3(Ń–≥Ųň„ ĹľīŅ…)°£
°ĺīūįł°Ņ 3d24s2 Ńý∑Ĺ◊Ó√‹ ∑÷◊” KCl°ĘCaOĺý–ő≥…ĶńĹŠĻĻŌŗň∆ĶńņŽ◊”ĺßŐŚ£¨∂ÝK+įŽĺ∂īů”ŕCa2+£¨K+ĶÁļ…ŃŅ–°”ŕCa2+Ķń°ĘCl-įŽĺ∂īů”ŕO2-£¨Cl-ĶÁļ…ŃŅ–°”ŕO2-Ķń£¨Ļ KCl ĺßłŮń‹–°”ŕCaOĶńĺßłŮń‹ O>N>C CaTiO3 ![]() 4:3
4:3 ![]()
°ĺĹ‚őŲ°Ņ£®1£©ĽýŐ¨Ó—‘≠◊”ĶńľŘĶÁ◊”ŇŇ≤ľ Ĺő™3d24s2°£”…Ĺū ŰÓ—ĺßįŻ ĺ“‚ÕľľįĺßįŻ≤ő żŅ…÷™£¨Ĺū ŰÓ—ő™Ńý∑Ĺ◊Ó√‹∂—Ľż°£
£®2£©”√»Ř»ŕĶń√ĺ‘ŕŽ≤∆Ý÷–ĽĻ‘≠TiCl4Ņ…Ķ√ĶĹ∂ŗŅ’Ķńļ£√ŗÓ—°£“—÷™TiCl4‘ŕÕ®≥£«ťŅŲŌ¬ «őř…ę“ļŐŚ£¨»ŘĶ„ő™-23°ś£¨∑–Ķ„ő™136°ś£¨∆š»ŘĶ„ļÕ∑–Ķ„ĹŌĶÕ£¨ňý“‘TiCl4ő™∑÷◊”ĺßŐŚ°£
£®3£©KClļÕCaO”ŽNaClĺßŐŚĹŠĻĻŌŗň∆£¨ŃĹ÷÷ņŽ◊”ĺßŐŚĶńĺßłŮń‹Ķńīů–°”…∆šņŽ◊”ľŁĶń«Ņ∂»ĺŲ∂®£¨∂ÝņŽ◊”ľŁĶń«Ņ∂»”ŽņŽ◊”įŽĺ∂ļÕņŽ◊”ĶÁļ… żŃĹłŲ∑Ĺ√śĻ≤Õ¨ĺŲ∂®°£KClĺßłŮń‹–°”ŕCaOĶń‘≠“Ú «£ļKCl°ĘCaOĺý–ő≥…Ķń «ĹŠĻĻŌŗň∆ĶńņŽ◊”ĺßŐŚ£¨∂ÝK+įŽĺ∂īů”ŕCa2+£¨K+ĶÁļ…ŃŅ–°”ŕCa2+Ķń°ĘCl-įŽĺ∂īů”ŕO2-£¨Cl-ĶÁļ…ŃŅ–°”ŕO2-Ķń£¨Ļ KClĺßłŮń‹–°”ŕCaOĶńĺßłŮń‹°£Õ¨“Ľ÷‹∆ŕĶń÷ų◊Ś‘™ňōī”◊ůĶĹ”“£¨ĶÁłļ–‘“ņīő‘Ųīů£¨ňý“‘C°ĘN°ĘO‘™ňōĶńĶÁłļ–‘”…īůĶĹ–°Ķńň≥–Ú «O>N>C°£
(4)”…ł∆Ó—ŅůĺßŐŚĶńĹŠĻĻ ĺ“‚ÕľŅ…÷™£¨ł√ĺßįŻńŕ”–1łŲł∆‘≠◊”£¨—ű‘≠◊”Ķń żńŅő™![]() £¨Ó—‘≠◊”Ķń żńŅő™
£¨Ó—‘≠◊”Ķń żńŅő™![]() £¨ňý“‘ł√ĺßŐŚĶńĽĮ—ß Ĺő™CaTiO3°£“—÷™‘≠◊”◊ÝĪÍő™A(0£¨0£¨0)°ĘB(0£¨1/2£¨0)£¨‘ÚAő™◊ÝĪÍ‘≠Ķ„£¨“Úő™BőĽ”ŕĺßįŻy÷Š…Ō«“ő™ņ‚Ķń÷––ń°Ęł∆ņŽ◊”‘ŕĺßįŻĶńŐŚ–ń£¨ňý“‘CaņŽ◊”Ķń‘≠◊”◊ÝĪÍő™
£¨ňý“‘ł√ĺßŐŚĶńĽĮ—ß Ĺő™CaTiO3°£“—÷™‘≠◊”◊ÝĪÍő™A(0£¨0£¨0)°ĘB(0£¨1/2£¨0)£¨‘ÚAő™◊ÝĪÍ‘≠Ķ„£¨“Úő™BőĽ”ŕĺßįŻy÷Š…Ō«“ő™ņ‚Ķń÷––ń°Ęł∆ņŽ◊”‘ŕĺßįŻĶńŐŚ–ń£¨ňý“‘CaņŽ◊”Ķń‘≠◊”◊ÝĪÍő™![]() °£
°£
£®5£©ĘŔ”…ÕľŅ…÷™£¨¶ń-FeĺßįŻő™ŐŚ–ńŃĘ∑Ĺ£¨∆šŇšőĽ żő™8£¨¶Ń-FeĺßįŻő™ľÚĶ•ŃĘ∑Ĺ£¨∆šŇšőĽ żő™6£¨ňý“‘£¨ŃĹ÷÷ĺßŐŚĺßįŻ÷–Őķ‘≠◊”ĶńŇšőĽ ż÷ģĪ»ő™4:3°£
Ęŕ¶ń-FeĺßįŻő™ŐŚ–ńŃĘ∑Ĺ£¨»ŰFe‘≠◊”įŽĺ∂ő™rpm£¨‘ÚĺßįŻĶńĪŖ≥§ő™![]() °£NAĪŪ ĺįĘ∑Łľ”Ķ¬¬ř≥£ żĶń÷Ķ£¨¶ń-FeĺßįŻ÷–”–2łŲ‘≠◊”£¨NAłŲ¶ń-FeĺßįŻĶń÷ ŃŅļÕŐŚĽż∑÷Īūő™56
°£NAĪŪ ĺįĘ∑Łľ”Ķ¬¬ř≥£ żĶń÷Ķ£¨¶ń-FeĺßįŻ÷–”–2łŲ‘≠◊”£¨NAłŲ¶ń-FeĺßįŻĶń÷ ŃŅļÕŐŚĽż∑÷Īūő™56![]() ļÕ
ļÕ![]() £¨‘Ú¶ń-FeĶ•÷ Ķń√‹∂»ő™
£¨‘Ú¶ń-FeĶ•÷ Ķń√‹∂»ő™![]() g/cm3°£
g/cm3°£


 ŐžŐžŌÚ…ŌŅő ĪÕ¨≤Ĺ—ĶŃ∑ŌĶŃ–īūįł
ŐžŐžŌÚ…ŌŅő ĪÕ¨≤Ĺ—ĶŃ∑ŌĶŃ–īūįł —ŰĻ‚ŅőŐ√Õ¨≤ĹŃ∑ŌįŌĶŃ–īūįł
—ŰĻ‚ŅőŐ√Õ¨≤ĹŃ∑ŌįŌĶŃ–īūįł
| ńÍľ∂ | łŖ÷–Ņő≥Ő | ńÍľ∂ | ≥ű÷–Ņő≥Ő |
| łŖ“Ľ | łŖ“Ľ√‚∑—Ņő≥ŐÕ∆ľŲ£° | ≥ű“Ľ | ≥ű“Ľ√‚∑—Ņő≥ŐÕ∆ľŲ£° |
| łŖ∂Ģ | łŖ∂Ģ√‚∑—Ņő≥ŐÕ∆ľŲ£° | ≥ű∂Ģ | ≥ű∂Ģ√‚∑—Ņő≥ŐÕ∆ľŲ£° |
| łŖ»ż | łŖ»ż√‚∑—Ņő≥ŐÕ∆ľŲ£° | ≥ű»ż | ≥ű»ż√‚∑—Ņő≥ŐÕ∆ľŲ£° |
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°Ņń≥»‹“ļ÷–ĹŲļ¨”–“Ľ÷÷»‹÷ £¨»Ű‘ŕł√»‹“ļ÷–ľ”»ŽBaCl2»‹“ļ≥ŲŌ÷į◊…ę≥ŃĶŪ‘Ŕľ”Ō°HNO3≥ŃĶŪ≤ĽŌŻ ߣ¨‘Úł√»‹“ļ≤ĽŅ…ń‹ļ¨”–Ķń»‹÷ «£® £©
A.AgNO3
B.CuSO4
C.K2SO3
D.Na2CO3
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°Ņń≥Õ¨—ßĹę 0.1mol/L Ķń K2SO4 »‹“ļ V 1 L ”Ž 0 . 2 mo l / L Ķń Al2(SO4)3»‹“ļ V 2 L ĽžļŌ£¨‘Ŕľ”»Ž V 3 L ’ŰŃůňģ£¨ľŔ∂®»‹“ļ ◊‹ŐŚĽż V ◊‹ =V1 +V 2 +V 3 £ģ≤Ę≤‚Ķ√ĽžļŌ“ļ÷–»ż÷÷ņŽ◊”őÔ÷ ĶńŃŅŇ®∂» ∑÷Īūő™£ļK£ę£ļ0 .1 mo l /L £¨Al3£ę£ļ0 .1 mo l /L£¨SO42£≠£ļ0 . 2 mo l / L £¨ ‘Ú Ō¬ Ń– Ň– ∂Ō ’ż »∑ Ķń «£® £©
A. “Ľ∂® « 2L K2SO4 »‹“ļļÕ 1L Al2(SO4)3»‹“ļĽžļŌ£¨‘Ŕľ” 1L ’ŰŃůňģ
B. ĽžļŌ“ļ÷–K£ęŇ®∂»”ŽAl3£ęŇ®∂» ż÷Ķ÷ģļÕīů”ŕSO42£≠Ň®∂» ż÷Ķ
C. »ż÷÷“ļŐŚŐŚĽżĪ»ő™ V1£ļV2£ļV3=2£ļ1£ļ1
D. ĽžļŌ“ļ÷– K2SO4 őÔ÷ ĶńŃŅĶ»”ŕ Al2(SO4)3őÔ÷ ĶńŃŅĶń“ĽįŽ
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°ŅŌ÷”–14.4gCOļÕCO2ĶńĽžļŌ∆ÝŐŚ£¨‘ŕĪÍ◊ľ◊īŅŲŌ¬∆šŐŚĽżő™ 8.96L°£«ŽĽōīūŌ¬Ń–ő Ő‚£ļ
£®1£©ł√ĽžļŌ∆ÝŐŚĶń∆Ĺĺýń¶∂Ż÷ ŃŅ ő™ _____°£
£®2£©ł√ĽžļŌ∆ÝŐŚ ÷–Őľ‘≠◊”ĶńłŲ ż ő™ _____£Ľ£®”√N AĪŪ ĺįĘ∑Łľ”Ķ¬¬ř≥£ żĶń÷Ķ£©
£®3£©Ĺęł√ĽžļŌ∆ÝŐŚ“ņīőÕ®Ļż»ÁŌ¬Õľňý ĺ◊į÷√£¨◊Óļů ’ľĮ‘ŕ∆Ý«Ú÷–£®ŐŚĽż‘ŕĪÍ◊ľ◊īŅŲŌ¬≤‚∂®£© £ģ

∆Ý«Ú÷– ’ľĮĶĹĶń∆ÝŐŚĶńń¶∂Ż÷ ŃŅ _____£Ľ
Ęŕ ∆Ý«Ú÷– ’ľĮĶĹĶń∆ÝŐŚĶńĶÁ◊”◊‹ żő™ _____£Ľ£®”√ NAĪŪ ĺįĘ∑Łľ”Ķ¬¬ř≥£ żĶń÷Ķ £©
ĘŘ ĪÍŅŲŌ¬∆Ý«Ú÷– ’ľĮĶĹĶń∆ÝŐŚĶńŐŚ Ľżő™ _____L°£
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°ŅŐŗ(Sb)ľį∆šĽĮļŌőÔ‘ŕĻ§“Ķ…Ō”––Ū∂ŗ”√Õĺ°£“‘Ľ‘ŐŗŅů£®÷ų“™≥…∑÷ő™Sb2S3£¨ĽĻļ¨”–PbS°ĘAs2S3°ĘCuO°ĘSiO2Ķ»£©ő™‘≠ŃŌ÷∆ĪłĹū ŰŐŗĶńĻ§“’Ńų≥Ő»ÁÕľňý ĺ£ļ
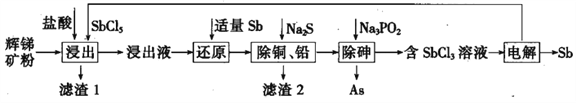
“—÷™£ļĘŔ ĹĢ≥Ų“ļ÷–≥żļ¨ĻżŃŅ—őňŠļÕSbCl5÷ģÕ‚£¨ĽĻļ¨”–SbCl3°ĘPbCl2°ĘAsCl3°ĘCuCl2Ķ»£Ľ
Ęŕ≥£ő¬Ō¬£ļKsp(CuS)=1.27°Ń10-36£¨Ksp(PbS)=9.04°Ń10-29£Ľ
ĘŘ»‹“ļ÷–ņŽ◊”Ň®∂»–°”ŕĶ»”ŕ1.0°Ń10-5mol°§L-1 Ī£¨»Ōő™ł√ņŽ◊”≥ŃĶŪÕÍ»ę°£
£®1£©¬ň‘Ł1÷–≥żŃňS÷ģÕ‚£¨ĽĻ”–___________£®ŐÓĽĮ—ß Ĺ£©°£
£®2£©°įĹĢ≥Ų°Ī Ī£¨Sb2S3∑Ę…ķ∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺő™_________________°£
£®3£©°įĽĻ‘≠°Ī Ī£¨ĪĽSbĽĻ‘≠ĶńőÔ÷ ő™_____________£®ŐÓĽĮ—ß Ĺ£©°£
£®4£©≥£ő¬Ō¬£¨°į≥żÕ≠°Ę«¶°Ī Ī£¨Cu2+ļÕPb2+ĺý≥ŃĶŪÕÍ»ę£¨īň Ī»‹“ļ÷–Ķńc(S2-)≤ĽĶÕ”ŕ______£Ľňýľ”Na2S“≤≤Ľ“ňĻż∂ŗ£¨∆š‘≠“Úő™_____________°£
£®5£©°į≥ż…ť°Ī Ī”–H3PO3…ķ≥…£¨ł√∑ī”¶ĶńĽĮ—ß∑Ĺ≥Ő Ĺő™________________°£
£®6£©°įĶÁĹ‚°Ī Ī£¨ĪĽ—űĽĮĶńSb‘™ňō”ŽĪĽĽĻ‘≠ĶńSb‘™ňōĶń÷ ŃŅ÷ģĪ»ő™_______°£
£®7£©“Ľ÷÷ÕĽ∆∆īęÕ≥ĶÁ≥ō…Ťľ∆ņŪńÓĶń√ĺ-Őŗ“ļŐ¨Ĺū ŰīĘń‹ĶÁ≥ōĻ§◊ų‘≠ņŪ»ÁÕľňý ĺ£ļ

ł√ĶÁ≥ō”…”ŕ√‹∂»Ķń≤ĽÕ¨£¨‘ŕ÷ōѶ◊ų”√Ō¬∑÷ő™»ż≤„£¨Ļ§◊ų Ī÷–ľš≤„»Ř»ŕ—őĶń◊ť≥…≤ĽĪš°£≥šĶÁ Ī£¨C1-ŌÚ_____£®ŐÓ°į…Ō°ĪĽÚ°įŌ¬°Ī£©“∆∂Į£Ľ∑ŇĶÁ Ī£¨’żľęĶńĶÁľę∑ī”¶ Ĺő™________°£
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°ŅŌ¬Ń–ňĶ∑®’ż»∑Ķń «£® £©
A. ĽĮ—ß∆Ĺļ‚’żŌÚ∑Ę…ķ“∆∂Į Ī£¨∆Ĺļ‚≥£ żK÷Ķ“Ľ∂®‘Ųīů
B. HS£≠ĶńĶÁņŽ∑Ĺ≥Ő Ĺ£ļHS£≠+H2O![]() S2£≠+H3O+
S2£≠+H3O+
C. ”…ňģĶÁņŽ≥ŲĶńc(H+)£Ĺ1°Ń10£≠13mol/LĶń»‹“ļ÷–£¨Ņ…ń‹īůŃŅĻ≤īśĶńņŽ◊”£ļFe3+°ĘK+°ĘNH4+°Ę SO42£≠°ĘCl£≠°ĘClO£≠
D. AlCl3»‹“ļ”ŽNa2CO3»‹“ļĽžļŌ∑Ę…ķ∑ī”¶£ļ2Al3++3CO32£≠£ĹAl2(CO3)3°ż
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°ŅŌ¬Ń–őÔ÷ ÷–£¨ Ű”ŕĻ≤ľŘĽĮļŌőÔĶń «£® £©
A.CO2B.NH4ClC.CaCl2D.NaCl
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°Ņ»ÁÕľ£¨Őķ∆¨°ĘÕ≠∆¨ļÕCuSO4»‹“ļŅ…“‘ĻĻ≥…‘≠ĶÁ≥ōĽÚĶÁĹ‚≥ō£¨Ō¬Ń–ňĶ∑®’ż»∑Ķń «£® £©

A. ĻĻ≥…‘≠ĶÁ≥ō Ī£¨Cuľę∑ī”¶ Ĺő™Cu£≠2e£≠£ĹCu2+
B. ĻĻ≥…ĶÁĹ‚≥ō Ī£¨Cuľę÷ ŃŅŅ…ń‹ľű…Ŕ“≤Ņ…ń‹‘Ųľ”
C. ĻĻ≥…ĶÁĹ‚≥ō Ī£¨Feľę÷ ŃŅ“Ľ∂®ľű…Ŕ
D. ĻĻ≥…Ķń‘≠ĶÁ≥ōĽÚĶÁĹ‚≥ō‘ŕĻ§◊ų ĪĶń∑ī”¶‘≠ņŪ“Ľ∂®≤ĽÕ¨
≤ťŅīīūįłļÕĹ‚őŲ>>
Ņ∆ńŅ£ļłŖ÷–ĽĮ—ß ņī‘ī£ļ Ő‚–Õ£ļ
°ĺŐ‚ńŅ°Ņ»ÁÕľ∑÷ĪūĪŪ ĺ…ķőÔŐŚńŕĶń…ķőÔīů∑÷◊”Ķń≤Ņ∑÷ĹŠĻĻń£ ĹÕľ£¨ĺ›ÕľĽōīūŌ¬Ń–ő Ő‚:

£®1£©Õľľ◊÷–Ķń»ż÷÷őÔ÷ ĶńĽýĪĺĶ•őĽ∂ľ «___________£¨∆š÷– Ű”ŕ∂ĮőÔŌłįŻīĘń‹őÔ÷ Ķń «___________°£’‚»ż÷÷őÔ÷ ÷–£¨‘ŕĻ¶ń‹…Ō”ŽŃŪÕ‚ŃĹ÷÷Ĺō»Ľ≤ĽÕ¨Ķń «______________________°£
£®2£©Õľ““ĽĮļŌőÔ «∑ő—◊ňę«Úĺķ“ŇīęőÔ÷ Ķń“Ľ≤Ņ∑÷£¨∆šĽýĪĺĶ•őĽ «__________£¨Ņ…”√Õľ÷–◊÷ńł_______ĪŪ ĺ£¨łųĽýĪĺĶ•őĽ÷ģľš «Õ®Ļż___________£®ŐÓĘŔ°ĘĘŕĽÚĘŘ£©Ń¨Ĺ”∆ūņīĶń°£
£®3£©ÕľĪŻňý ĺĽĮļŌőÔĶń√Ż≥∆ «___________£¨ «”…___________÷÷įĪĽýňŠĺ≠___________Ļż≥Ő–ő≥…Ķń£¨Õ—»•ňģ÷–Ķń«‚‘™ňōņī◊‘___________£¨Ń¨Ĺ”įĪĽýňŠ÷ģľšĶńĽĮ—ßľŁ «___________£®ŐÓĽĮ—ßľŁĹŠĻĻ£©°£
≤ťŅīīūįłļÕĹ‚őŲ>>
Ļķľ —ß–£”Ň—° - Ń∑Ōį≤ŠŃ–ĪŪ - ‘Ő‚Ń–ĪŪ
ļĢĪĪ °Ľ•Ń™ÕÝő•∑®ļÕ≤ĽŃľ–ŇŌĘĺŔĪ®∆ĹŐ® | ÕÝ…Ō”–ļ¶–ŇŌĘĺŔĪ®◊®«Ý | ĶÁ–Ň’©∆≠ĺŔĪ®◊®«Ý | …śņķ ∑–ťőř÷ų“Ś”–ļ¶–ŇŌĘĺŔĪ®◊®«Ý | …ś∆ů«÷»®ĺŔĪ®◊®«Ý
ő•∑®ļÕ≤ĽŃľ–ŇŌĘĺŔĪ®ĶÁĽį£ļ027-86699610 ĺŔĪ®” Ōš£ļ58377363@163.com