
��ɫ����Һ�п��ܺ�����������:K+��Al3+��Fe3+��Ba2+��NO3����SO42����HCO32����Cl-,ȡ����Һ��������ʵ��:
������ɫʯ����ֽ������Һ,��ֽ�Ժ�ɫ;
��ȡԭ��Һ����,����ͭƬ��ϡ���Ṳ��,������ɫ����,������������������Ϊ����ɫ;
��ȡԭ��Һ����,���백ˮ�а�ɫ��������,�������������ˮ,��������ʧ;
��ȡԭ��Һ����,�����Ȼ�����Һ������ɫ����;
��ȡʵ��ܺ�ij�����Һ,������������Һ������ɫ����,�ټ��������ϡ����,��������ʧ��
��ش���������:
(1)��������ʵ���ж�ԭ��Һ���������ӿ϶����ڵ���������������������,�϶������ڵ����� ��
(2)д����ڢ�����ʵ���йص����ӷ���ʽ:
�� , ���� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ba(OH)2��Һ����μ���ϡ���ᣬ������������⣺
(1)д����Ӧ�����ӷ���ʽ�� ��
(2)������������£����ӷ���ʽ�� (1)��ͬ���� (�����)��
A����NaHSO4��Һ����μ���Ba(OH)2��Һ����Һ������
B����NaHSO4��Һ����μ���Ba(OH)2��Һ��SO42��ǡ����ȫ����
C����NaHSO4��Һ����μ���Ba(OH)2��Һ������
(3)����������ϡ����ֱ�����������������л����Һ�ĵ�������(�õ���ǿ��I��ʾ)�ɽ��Ƶ�����ͼ�е� (�����)���߱�ʾ��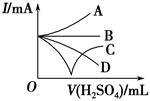
(4)����һ����⻬������С��������Ba(OH)2��Һ���룬��ͼ��ʾ������ձ��л���ע����Ba(OH)2��Һ���ܶȵ�ϡ������ǡ����ȫ��Ӧ���ڴ�ʵ������У�С�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij���������̿�MnO2Լ70%��Al2 O3������п��ZnSԼ80%��FeS����ͬ����MnO2����Zn���ɵ��ԭ�ϣ����������£�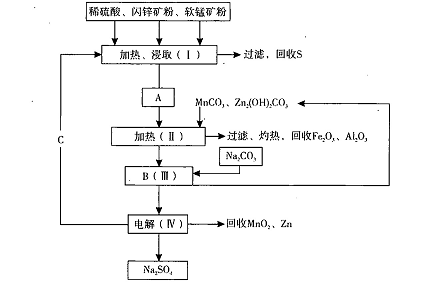
��֪����A��MnSO4��ZnSO4��Fe2(SO4)3��Al2(SO4)3�Ļ��Һ��
��IV�еĵ�ⷴӦʽΪMnSO4+ZnSO4+2H2O MnO2+Zn +2H2SO4��
MnO2+Zn +2H2SO4��
��1��A�����ڻ�ԭ������� ��
��2������MnCO3��Zn2(OH)2CO3�������� ��C�Ļ�ѧʽ�� ��
��3���������г��õ�Na2SO4��S�ȸ���Ʒ�⣬���ɵõ��ĸ���Ʒ�� ��
��4������ƷS�����������ᣬת�������ǣ�S��SO2��SO3��H2SO4��д���ڶ���ת���Ļ�ѧ����ʽ ��
��5��Ҫ��Na2SO4��Һ�еõ�â���� Na2SO4��10H2O��������еIJ���������Ũ���� ��
���ˡ�ϴ�ӡ�����ȡ�
��6��������MnO2��Zn�ĽǶȼ��㣬���̿����п��Ͷ�ϵ������ȴ�Լ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ũҩ��Ⱦ�ϡ��ӡ��軯��Լ�����ɫ�ȡ���ζ�ķ�ˮ�����û�ѧ���������д��������õ������������ࣨ��Һ�ȡ�������ơ��������Ƶȣ������ࣨ��������������������⡢������صȣ���һ������ʵ���������������������о綾���軯���CN�����ķ�ˮ���ڼ��������£�pH��8.5��11���������ɽ��軯����CN������Ϊֻ��������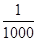 �������Σ���CNO������
����������CNO������
��1��д����CN����ˮ�������������������ε����ӷ���ʽ��________________________________________________________________��
��2������CNO���ķ�ˮ����ͨ����������ʹCNO��ת��Ϊ�������壬д�������Ӧ�����ӷ���ʽ��______________________________________________��
��3������Һ�Ȳ���ĵ���������Ư�۴�����CN���ķ�ˮ������������ΪCNO���������ӷ���ʽΪ__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��Һ���ܺ���Na����K����Mg2����Cu2���������Ӽ�MnO4����SiO32����AlO2����CO32����HCO3����SO42����Cl���������ӣ���֪���ٸ���Һ����ɫ���ھ��ⶨ��Һ��pH��12����ȡ������Һ������100 mL 2 mol��L��1ϡ��������ữ���а�ɫ�������ɣ����õ�һ����ɫ��ζ�����壬������ʹ����ʯ��ˮ(����)����ǡ����ữ�����Һ���ˣ��õ���Һ�ס�
(1)�ɢ٢ڢۿ��жϣ�ԭ��Һ��һ�������ڵ�������________��һ�����ڵ�������________��
(2)����Һ�ֳ����ȷݣ�һ������μ��백ˮ�������а�ɫ��״������˵��ԭ��Һ��һ����________(�����ӷ���)���տ�ʼ���백ˮʱ��û�г���������ԭ����____________________________________(�����ӷ���ʽ��ʾ)����һ���м���������Ba(NO3)2��Һ���а�ɫ�������ɣ�˵��ԭ��Һ��һ����________(�����ӷ���)�����˵õ���Һ�ҡ�
(3)����Һ���м���������AgNO3��Һ�����ˡ�ϴ�ӡ�����ù���26.5 g����ԭ��Һ���Ƿ���Cl����________(��ǡ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������A��B��C��D��E�����ǵ������ӿ�����Na����NH4+��Cu2����Ba2����Al3����Ag����Fe3���������ӿ�����Cl����NO3-��SO42-��CO32-����֪��
�������ξ�����ˮ��ˮ��Һ��Ϊ��ɫ��
��D����ɫ��Ӧ�ʻ�ɫ��
��A����Һ�����ԣ�B��C��E����Һ�����ԣ�D����Һ�ʼ��ԡ�
�������������ε���Һ�зֱ����Ba(NO3)2��Һ��ֻ��A��C����Һ������������
�������������ε���Һ�У��ֱ���백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ��
�ް�A����Һ�ֱ���뵽B��C��E����Һ�У��������ɲ�����ϡ����ij�����
��ش��������⣺
��1���������У�һ��û�е���������____________��������������ͬ�������εĻ�ѧʽ��__________________��
��2��D�Ļ�ѧʽΪ__________________��D��Һ�Լ��Ե�ԭ����_________________(�����ӷ���ʽ��ʾ)��
��3��A��C����Һ��Ӧ�����ӷ���ʽ��______________________________________��
E�Ͱ�ˮ��Ӧ�����ӷ���ʽ��____________________________________________��
��4����Ҫ����B�������������ӣ���ȷ��ʵ�鷽����______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪ 0.4 mol Һ̬�º�����H2O2��Ӧ���ɵ�����ˮ����ʱ�ų�256.64 kJ��������
(1)д���º�H2O2��Ӧ���Ȼ�ѧ����ʽ: ��
(2)��֪H2O(l)=H2O(g) ��H="+44" kJ/mol,��16 gҺ̬��������˫��ˮ��Ӧ���ɵ�����Һ̬ˮʱ,�ų��������� ��
(3)������ӦӦ���ڻ���ƽ���,���ͷų����������Ϳ��ٲ�������������,����һ����ͻ�����ŵ��� ��
(4)�����������Һ��ͨ��һ�����ʵ����İ�����������,д����Ӧ�����ӷ���ʽ: ,�÷�Ӧ�Ļ�ԭ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ӵ�ʳ���м���ĵ������һ�ְ�ɫ�ᾧ��ĩ�����ܽ�����¶�Ӱ��ܴ������¶ȵ����߶����������º��ȶ��������������µ������һ�ֽ�ǿ��������������⻯��Ȼ�ԭ�����ʷ�����Ӧ��
��1��Ϊ����ijʳ�����Ƿ���KIO3��ijͬѧȡʳ����Ʒ��ȫ�ܽ���ˮ�У�Ȼ������������ữ�ĵ���KI��Һ�������Һ����ɫ����Ӧ�����ӷ���ʽΪ ��
��2��KIO3Ҳ���õ��ķ����Ƶã���ԭ������ʯīΪ�������Բ����Ϊ��������һ���¶Ⱥ͵����µ��KI��Һ���ܷ�Ӧ����ʽΪKI+3H2O=KIO3+3H2�����������ĵ缫��Ӧʽ�ֱ�Ϊ������ ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��4���ƵĻ�����W��X��Y��Z������֮��������¹�ϵ��
��W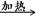 X��H2O��CO2��
X��H2O��CO2��
��Z��CO2�D��X��O2
��Z��H2O�D��Y��O2��
��X��Ca(OH)2�D��Y��CaCO3��
�Իش��������⣺
(1)W��X��Y��Z�Ļ�ѧʽ�ֱ��ǣ�W��________��X��________��Y��________��Z��________��
(2)����4����ѧ��Ӧ������������ԭ��Ӧ����________(�Ӧ���)����Ӧ����������________(д��ѧʽ)����ԭ����________(д��ѧʽ)��
(3)���ܷ�Ӧ����Һ�н��У�д�������ӷ���ʽ�Լ����ø����ӷ���ʽ��ʾ����һ����ѧ��Ӧ�Ļ�ѧ����ʽ�������ӷ���ʽ��__________________________���ڻ�ѧ����ʽ��_______________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com