
ij���������̿�MnO2Լ70%��Al2 O3������п��ZnSԼ80%��FeS����ͬ����MnO2����Zn���ɵ��ԭ�ϣ����������£�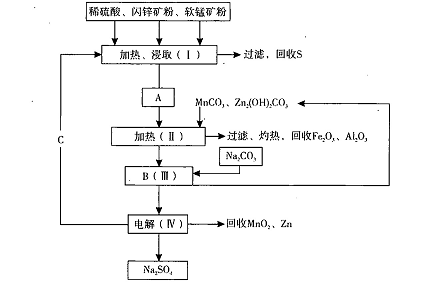
��֪����A��MnSO4��ZnSO4��Fe2(SO4)3��Al2(SO4)3�Ļ��Һ��
��IV�еĵ�ⷴӦʽΪMnSO4+ZnSO4+2H2O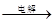 MnO2+Zn +2H2SO4��
MnO2+Zn +2H2SO4��
��1��A�����ڻ�ԭ������� ��
��2������MnCO3��Zn2(OH)2CO3�������� ��C�Ļ�ѧʽ�� ��
��3���������г��õ�Na2SO4��S�ȸ���Ʒ�⣬���ɵõ��ĸ���Ʒ�� ��
��4������ƷS�����������ᣬת�������ǣ�S��SO2��SO3��H2SO4��д���ڶ���ת���Ļ�ѧ����ʽ ��
��5��Ҫ��Na2SO4��Һ�еõ�â���� Na2SO4��10H2O��������еIJ���������Ũ���� ��
���ˡ�ϴ�ӡ�����ȡ�
��6��������MnO2��Zn�ĽǶȼ��㣬���̿����п��Ͷ�ϵ������ȴ�Լ�� ��
��1��MnSO4
(2)������Һ��p H,ʹFe3+��Al3+���ɳ��� H2SO4
��3��Fe2O3��Al2O3
(4) 2SO2+O2 2SO3
2SO3
(5) ��ȴ�ᾧ
��6��1��1(��1.03:1)
��������������Ƚ���Ϣ1��֪Mn���ϼ۽��ͣ����ڻ�ԭ�����ΪMnSO4���ɹ������̷�������MnCO3��Zn2(OH)2CO3��������������Һ��p H,ʹFe3+��Al3+���ɳ�������C�Ļ�ѧʽΪH2SO4�����ѵó��õ��ĸ���Ʒ����Fe2O3��Al2O3���������̿����п��������ֱ�Ϊx ��y�����ݢڿ�֪MnO2��Zn�����ʵ���֮��Ϊ1:1����0.7x g/87g/mol:0.8y g/97g/mol=1:1,���x/y=1.03:1.
���㣺������ԭ��Ӧ��Ԫ�ػ�����֪ʶ�����ʵķ�����ᴿ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ɫ��ҺX����Na+��Ag+��Ba2+��Al3+��[Al(OH)4]--�� MnO4����CO32-- ��SO42���е�������������ϣ�ȡ��Һ���������������飺���ѧ���
��1������A�ijɷ��ǣ�_________________������B�ijɷ���_____________
��2)X��Һ��һ�����ڵ������ǣ�____________________________
��3)д������ٷ�����Ӧ���������ӷ�Ӧ����ʽ��_________________________
��4��д��������γɰ�ɫ���������ӷ���ʽ��______________________
��5)д����ɫ�����ҵĿ�����ɣ�____________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ȡһ����������KMnO4��Һ���ν�������ʵ�飬�й������¼���£�
�ٵμ�����H2O2���Ϻ�ɫ��ȥ���������ݲ�����
���ټ���������PbO2���壬�����ܽ⣬��Һ�ֱ�Ϊ�Ϻ�ɫ��
�����ͨ��������SO2���塣
��ش��������⣺
(1)KMnO4��H2O2��PbO2��������ǿ������˳��Ϊ____________________��
(2)ʵ����е�����Ϊ_____________________________________________________��
(3)ʵ��١����з�Ӧ�����ӷ���ʽ�ֱ���__________��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������(ClO2)Ϊһ�ֻ���ɫ���壬�ǹ����Ϲ��ϵĸ�Ч�����ס����١���ȫ��ɱ����������
(1)��ҵ���Ʊ�ClO2�ķ�Ӧԭ��Ϊ2NaClO3��4HCl=2ClO2����Cl2����2H2O��2NaCl��
��Ũ�����ڷ�Ӧ����ʾ������������________��
| A��ֻ�л�ԭ�� | B����ԭ�Ժ����� |
| C��ֻ�������� | D�������Ժ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʳ���к���һ������þ���������ʣ��ӵ����е����ʧ��Ҫ���������ʡ�ˮ�֡������е������Լ����ա����ȶ�����ġ���֪��
�����ԣ�IO3-��Fe3����I2����ԭ�ԣ�S2O32-��I��
3I2��6OH��=5I����IO3-��3H2O
KI��I2 KI3
KI3
(1)ijѧϰС��Լӵ��ν���������ʵ�飺ȡһ����ij�ӵ���(���ܺ���KIO3��KI��Mg2����Fe3��)������������ˮ�ܽ⣬����ϡ�����ữ����������Һ��Ϊ3�ݡ���һ����Һ�еμ�KSCN��Һ���Ժ�ɫ���ڶ�����Һ�м�����KI���壬��Һ�Ե���ɫ����CCl4��ȡ���²���Һ���Ϻ�ɫ����������Һ�м�������KIO3����μӵ����Լ�����Һ����ɫ��
�ټ�KSCN��Һ�Ժ�ɫ���ú�ɫ������ (�û�ѧʽ��ʾ)��CCl4�����Ϻ�ɫ�������� (�õ���ʽ��ʾ)��
�ڵڶ�����Һ�м�������KI�����Ӧ�����ӷ���ʽΪ �� ��
(2)KI��Ϊ�ӵ����ʳ���ڱ�������У����ڿ��������������ã�������������ʧ��д����ʪ������KI��������Ӧ�Ļ�ѧ����ʽ�� ��
��I2����KI��Һ���ڵ��������£����Ƶ�KI3��H2O����������Ϊʳ�μӵ���Ƿ���ʣ� (��ǡ���)����˵�����ɣ� ��
(3)Ϊ����ӵ���(����KI)���ȶ��ԣ��ɼ��ȶ������ٵ����ʧ�������������п�����Ϊ�ȶ������� ��
| A��Na2S2O3 | B��AlCl3 |
| C��Na2CO3 | D��NaNO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
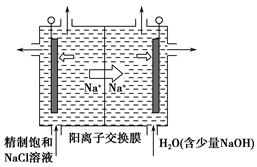
ij�������Թ�ҵ��ˮ�к���һ������Na����Al3����Fe3����Cu2����Cl�����ó�������ͼ��ʾ�Ĺ�������ͼ�����ó���������������ᡢ���ҵ�����еķ���м���ӷ�ˮ�����������Ȼ�������������NaCl����ͽ���ͭ�������˺ܺõ���ᾭ��Ч�档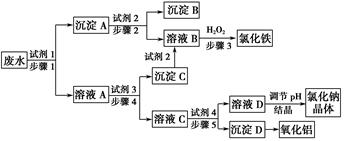
����д���пհף�
(1)ͼ���Լ�1��________���Լ�2��________��
(2)����1�Ͳ���2���õ��IJ���������________��
(3)����1��Ӧ�����ӷ���ʽΪ__________________________________��
(4)����3��Ӧ�����ӷ���ʽΪ__________________________________��
(5)�ӽ�ԼҩƷ�ͻ������濼�ǣ�����5��������Ӧ�����ӷ���ʽӦΪ____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һƿ��ɫ�������Һ�����п��ܺ�Na+��Mg2+��H+��Fe3+��CO32-��Cl-��Br-�е�һ�ֻ��֣�ȡ����Һ��������ʵ�飺
����PH��ֽ���飬������Һ��ǿ����
��ȡ������Һ������������CCl4���������Ƶ���ˮ����CCl4���ԳȺ�ɫ
�۽��ڵõ�����Һ�μ���������Һ���а�ɫ�������ɣ��μ�ϡ����������ܽ⡣
����ȡ������Һ����μ���ϡNaOH��Һ��ʹ��Һ��������ת��Ϊ���ԣ��ڵμӹ����м��μ���Ϻ���Һ�о���������
��������ʵ����ʵȷ�����ش�
��1������Һ�У��϶����ڵ������� ��
��2���϶������ڵ������� ��
��3�����ܴ��ڵ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ɫ����Һ�п��ܺ�����������:K+��Al3+��Fe3+��Ba2+��NO3����SO42����HCO32����Cl-,ȡ����Һ��������ʵ��:
������ɫʯ����ֽ������Һ,��ֽ�Ժ�ɫ;
��ȡԭ��Һ����,����ͭƬ��ϡ���Ṳ��,������ɫ����,������������������Ϊ����ɫ;
��ȡԭ��Һ����,���백ˮ�а�ɫ��������,�������������ˮ,��������ʧ;
��ȡԭ��Һ����,�����Ȼ�����Һ������ɫ����;
��ȡʵ��ܺ�ij�����Һ,������������Һ������ɫ����,�ټ��������ϡ����,��������ʧ��
��ش���������:
(1)��������ʵ���ж�ԭ��Һ���������ӿ϶����ڵ���������������������,�϶������ڵ����� ��
(2)д����ڢ�����ʵ���йص����ӷ���ʽ:
�� , ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ʣ���Һ�����ڰ�ˮ��������ܴ�������ᱵ�����������Ȼ��ƾ��壻�������̼�������泥���ƾ���Һ��
��1�����ڵ���ʵ���_____________________________________________________________��
��2���ܵ������_________________________________________________________________��
��3������ǿ����ʵ���___________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com