
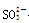 ��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�
��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�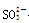
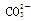 ��MnO4��
��MnO4�� 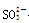 +Cl2+H2O ==2H++2Cl��+SO42��
+Cl2+H2O ==2H++2Cl��+SO42��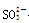 ��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�
��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�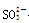
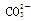 ���϶�û�е�������MnO4�� ��������е����ӷ���ʽ��Cl2+2Br��== Br2+2 Cl����
���϶�û�е�������MnO4�� ��������е����ӷ���ʽ��Cl2+2Br��== Br2+2 Cl����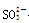 +Cl2+H2O ==2H++2Cl��+SO42����SO2����ͨ����з�̪��NaOH��Һ����ɫ����ȥ�����ܵ�ԭ��
+Cl2+H2O ==2H++2Cl��+SO42����SO2����ͨ����з�̪��NaOH��Һ����ɫ����ȥ�����ܵ�ԭ��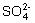 ��
��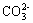 ��Ba2+��Ca2+��
��Ba2+��Ca2+��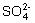 ��
��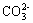 ��Fe2+��
��Fe2+�� (����������)��Fe3+��S2- ��Al3+��
(����������)��Fe3+��S2- ��Al3+�� ��S2- �ȵȣ�����������Һ�й��档
��S2- �ȵȣ�����������Һ�й��档

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
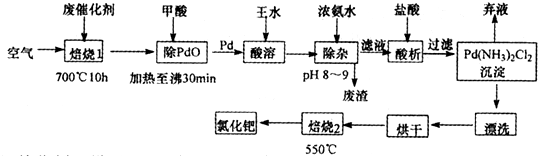
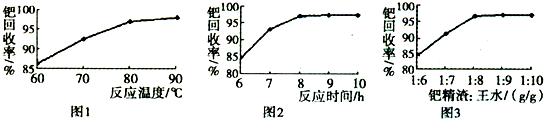
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
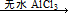 ArR+HX ��H<0��Ar��ʾ��������ij��ѧ��ȤС����ʵ�����������嶡�����е�90.70C)�����ᷴӦ�Ƶ��嶡���ȣ��е�500C)��������Fridel��Crafts��Ӧԭ���Ʊ����嶡�����ӣ��۵�990C)����Ӧ���̼�ʵ��װ������ͼ��ʾ��
ArR+HX ��H<0��Ar��ʾ��������ij��ѧ��ȤС����ʵ�����������嶡�����е�90.70C)�����ᷴӦ�Ƶ��嶡���ȣ��е�500C)��������Fridel��Crafts��Ӧԭ���Ʊ����嶡�����ӣ��۵�990C)����Ӧ���̼�ʵ��װ������ͼ��ʾ��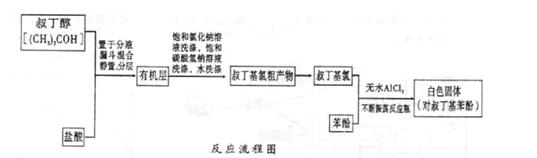
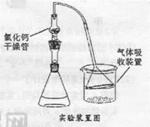
| ʵ�鷽�� | ʵ������ | �ܷ�˵��ˮ�� |
| �ٽ���ƿ�а���ͨ��HNO3�ữ��AgNO3��Һ | | |
| �ڽ����ð�������Һ����ˣ��ò����Թ��壬������ֳ����ݣ� �� | ���ݹ�����ܽ� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
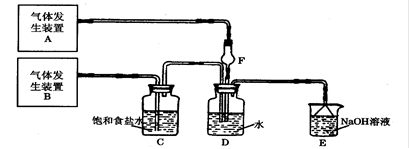
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����������A��B��֧�ྻ�Թ��У����á� | |
| ����2����A�Թ��м��������Ba(OH)2��Һ�����ã����ˡ� | |
| ����3��ȡ��������2�õ��������������������ᡣ | |
| ����4��ȡ��������2�õ��ĵ���Һ������ �� | |
| ����5����B�Թ��м��� �� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
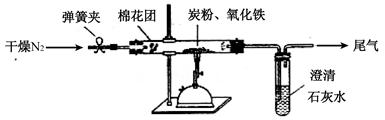
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
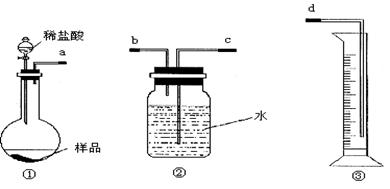
| A���ڵ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2���� |
| B����װ�â���ϡ���ỻ��ϡ���ᣬװ�â���ˮ���ɱ���NaHCO3��Һ |
| C����װ�â���ˮ���ɱ���Na2CO3��Һ |
| D���μ�����˹��� |
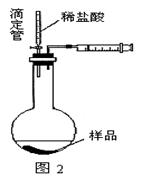
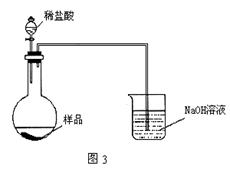
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
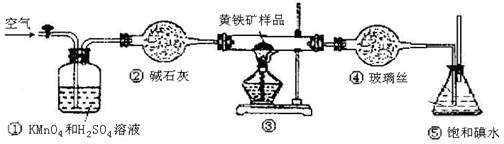
| �ζ����� | ����Һ������/mL | ������Һ�����/mL | |
| �ζ�ǰ | �ζ��� | ||
| ��һ�� | 20.00mL | 0.00 | 20.58 |
| �ڶ��� | 20.00mL | 0.22 | 20.20 |
| ������ | 20.00mL | 0.36 | 20.38 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com