
��12�֣����ǻ���Ƥ���Ǻϳ��㾫����Ҫԭ�ϣ���Ϊ�ϳ����ǻ���Ƥ���·��֮һ
��֪��
�Իش��������⣺
��1��������II�Ľṹ��ʽΪ��
��2��������II��������III���л���Ӧ����
��3��������III��������Һ�з�����Ӧ��ѧ����ʽ
��4���л���XΪ������IV��ͬ���칹�壬��֪�л���X�������ص㣺���DZ��Ķ�λȡ���������NaHCO3��Ӧ�ų����壬���ܷ���������Ӧ����д��������X�Ľṹ��ʽ
��5������˵����ȷ���ǣ� ��
| A��������I���Ȼ�����Һ����ɫ | B��������II����NaHCO3��Һ��Ӧ |
| C��1mol������IV��ȫȼ������9��5molO2 | D��1mol������III����3 mol H2��Ӧ |
��12�֣���1��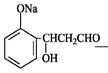
��2����ȥ��Ӧ
��3��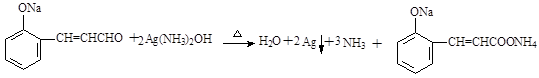
��4��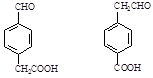
��5��A C
��6��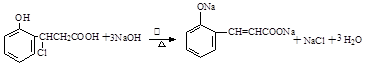 ��
��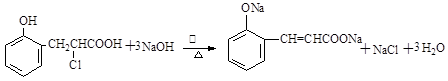
���������������1���ɻ�����III�Ľṹ��ʽ�жϣ�������II������ȥ��Ӧ�õ�������III�����Ի�����I����ȩ�����ӳɷ�Ӧ�õ�������II�����Ի�����II�Ľṹ��ʽΪ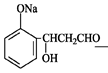
��2��������II��Ũ������ȵ������·�����ȥ��Ӧ���ɻ�����III��
��3��������III��������Һ�з���������Ӧ����ѧ����ʽΪ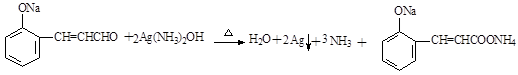 ��
��
��4������X�Ľṹ�ص㣬X�к��б��Ķ�λȡ�������Һ����Ȼ���ȩ����������IV�IJ����Ϲ���3��Cԭ�ӣ�������һCԭ�ӿ����Ȼ�������Ҳ����ȩ�����������X�Ľṹ��ʽ��2�֣��ֱ���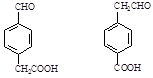
��5��A��������I�����к��з��ǻ��������Ȼ�����Һ����ɫ����ȷ��B��������II�в����Ȼ���������NaHCO3��Һ��Ӧ������C�����ݻ�����IV�Ľṹ��ʽ��֪1mol������IV��ȫȼ������9��5molO2����ȷ��D��������III�к��б�����̼̼˫����ȩ�����������������ӳɷ�Ӧ��1mol������III����5 mol H2��Ӧ������ѡAC��
��6�����ݻ�����IV�Ľṹ���ж��л���R��C9H9ClO3����Clԭ�ӵ�λ�ã����������ƵĴ���Һ�з�����ȥ��Ӧ���ɻ�����IV�����Ի�ѧ����ʽΪ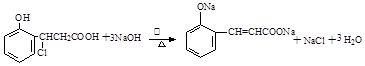 ��
��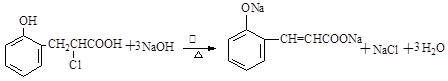
���㣺�����л���Ļ�ѧ���ʣ�ͬ���칹����жϣ���ѧ����ʽ����д����Ӧ���͵��ж�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�����10�֣�
2005���ŵ������ѧ���������ϩ�����ֽⷴӦ�о���������ͻ������3λ��ѧ�ҡ�ϩ�����ֽⷴӦʵ������һ��������ϩ����̼̼˫�������ŵĻ�λ��
�磺2CH2=CHCH2CH3 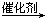 CH2��CH2+CH3CH2CH=CHCH2CH3��
CH2��CH2+CH3CH2CH=CHCH2CH3��
����֪������ȩ������һ�������¿����ȷ����ӳɷ�Ӧ��������ȥ��Ӧ��
�ֽ��Ա�ϩΪ�л�ԭ�ϣ��������з�Ӧ���Էֱ�ϳ���Ҫ�Ļ���ԭ��F��K����F��KΪԭ�Ͽɺϳ�һ����״�߷��ӻ�����M���仯ѧ���Ϊ(C12H20O4)n��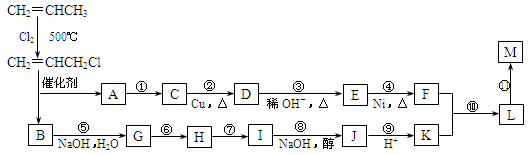
�ش��������⣺
43����Ӧ�ٵķ�Ӧ������_______________��
44����Ӧ�ޡ�������һ��Ӧ����HCl�ӳɣ��÷�Ӧ��_____���Ӧ��ţ��������һ����Ӧ��Ŀ����_____________________________________________________��
45������M�Ľṹ��ʽΪ��______________________________________��
46��д�����з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ�ࣺ_____________________________________________________________��
��Ӧ�⣺_____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
���ᡢ��ճ����ҵ�������ܼ�����̼������������DMC���ǽ������ܵ�������㷺��ע�Ļ�������ɫ������Ʒ������ӽṹ�к����ر��ԭ�ӻ���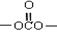 ������H2CO3Ҳ����д��
������H2CO3Ҳ����д�� �ṹ������������DMC�ĺϳɷ����ܶ࣬������������
�ṹ������������DMC�ĺϳɷ����ܶ࣬������������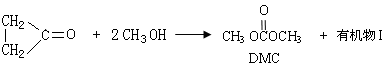
��1���л���I�Ľṹ��ʽΪ ��
��2��DMC�������·�Ӧ�٣�
�л���III����ʽΪ ��
�л���II�����������ˮ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ ��
��3��DMC������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ ��ע����������
��4���л���IV���л���III��һ��ͬ���칹�壬��IV������������������
A����һ�ַ����廯���� B������FeCl3������ɫ��Ӧ
C���ܷ���ˮ�ⷴӦ��Ҳ�ܷ���������Ӧ D�������ϵ�һ�ȴ���������
���л���IV�Ľṹ��ʽΪ ��
��5����DMC�ϳ�ɱ�����ά���·�����£�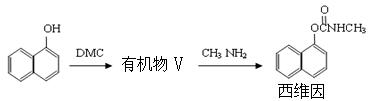
���������л���V�ķ�Ӧ�����ڷ�Ӧ�٣��л���V�Ľṹ��ʽΪ ��
1mol��ά�������� mol H2�����ӳɷ�Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
̼���������DMC)��һ�����͵���ɫ�л��ϳ��м��壬���������о���ʹ�ð�ȫ�����㡢��Ⱦ�١�����������ص㡣��֪һ�������£�̼��������ܷ������·�Ӧ��
��Ӧ�٣�
��Ӧ�ڣ�
��1��������III�ķ���ʽΪ ����Ӧ�ٺͷ�Ӧ����R�Ľṹ��ʽΪ ��
��2��DMC������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ ��
��3��̼������������ᱽ���� ���ڴ���������Ҳ�����ɻ������ͬʱ�õ�һ�ָ���ƷG�������й�G��˵����ȷ����_______��
���ڴ���������Ҳ�����ɻ������ͬʱ�õ�һ�ָ���ƷG�������й�G��˵����ȷ����_______��
A��G�������Ǽ�������
B�������G��Ϊͬ���칹��
C��һ�������£�G�ܷ���ˮ�ⷴӦ
D��G��������Cu(OH) 2��Ӧ���ɺ�ɫ����
��4����̼��������ϳ�ɱ�����ά���·�����£�
����������V�ķ�Ӧ�����ڷ�Ӧ�ڣ���V�Ľṹ��ʽΪ ��1mol��ά�������� mol H2�����ӳɷ�Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�л����(C13H18O2)��һ�����ϣ���ϳ�·����ͼ��ʾ������A����Է�������ͨ���������Ϊ56�����ĺ˴Ź���������ʾֻ������壻D���Է���������Ӧ���ڴ�������������1 mol D��2 mol H2��Ӧ���������ң����к�������-CH3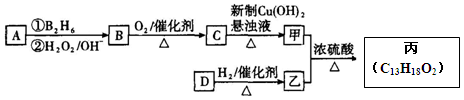
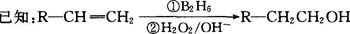
��1��A�Ľṹ��ʽΪ ���ҵķ���ʽΪ ��
��2��C������Cu(OH)2����Һ��Ӧ�Ļ�ѧ����ʽ_________________��
��3��D���������ŵ������� ��D�ж���ͬ���칹�壬��������������������ͬ��ͬ���칹���� �֣������������칹����
��4�������ҷ�Ӧ�Ļ�ѧ����ʽΪ ��
��5��д�����������������л���Ľṹ��ʽ �����һ�Ϊͬ���칹�壻����FeCl3��Һ����ɫ�����䱽���ϵ�һ�����ֻ�����֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
����֪�������ǻ�ͬʱ����ͬһ̼ԭ���ϵĽṹ�Dz��ȶ��ģ�����������ˮ��Ӧ��
���з���ʽΪC9H8O2Br2������M����һ�������¿ɷ�������һϵ�з�Ӧ��
��֪���л���A����Է�������Ϊ44���л���Iֻ��һ�ֽṹ����ʹ���CCl4��Һ��ɫ����ش��������⣺
��1��G��H�ķ�Ӧ������ ��
��2��H�еĹ����ŵ�����Ϊ ��D�Ľṹ��ʽΪ ��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
��A�� B�� ��
��H�� I�� ��
��4����G��Ϊͬ���칹�壬������������ȡ����������FeCl3��Һ��ɫ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��8�֣��㶹����һ����Ȼ���ϣ������ں��㶹��������ֲ���С���ҵ�ϳ���ˮ��ȩ���������ڴ��������¼��ȷ�Ӧ�Ƶã�
�������ɼױ�Ϊԭ�������㶹�ص�һ�ֺϳ�·�ߣ����ַ�Ӧ����������������ȥ��
��֪������Ϣ��
�� A�������ֲ�ͬ��ѧ�������⣻ �� B����FeCl3��Һ������ɫ��Ӧ��
�� ͬһ��̼ԭ�������������ǻ�ͨ�����ȶ�������ˮ�γ��ʻ���
��ش��������⣺
��1��A�Ļ�ѧ����Ϊ__________��
��2���㶹�صķ���ʽΪ_______���ɼױ�����A�ķ�Ӧ����Ϊ___________��
��3��B��ͬ���칹���к��б����Ļ���_____�֣������ں˴Ź���������ֻ������������_______�֣�
��4����B����C�Ļ�ѧ��Ӧ����ʽΪ___________��
��5��D��ͬ���칹���к��б����Ļ���______�֣����У����ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ����________��д�ṹ��ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��ϩ��Ϊԭ�ϣ��ϳ�ijЩ�߾����·�����£�
��֪��
��
����
��F����NaHCO3��Ӧ����CO2
������������⣺
��1��CH3CH=CHCH3�������� �� X�й����ŵ������� ��
��2��D��E�ķ�Ӧ����Ϊ�� ��
��3��д�����л�ѧ����ʽ��
A��B �� E �� ��Z��W ��
��Z��W ��
��4���߾���H�Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ�߶��ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
��10�֣�����������������ӵ�����ʽϿ��������ƣ�����β���ѳ�Ϊ��Ҫ�Ŀ�����Ⱦ�
 ��1��������ȼ������ʱ����Ӧ��N2��g����O2��g�� 2NO��g�����ǵ�������β���к���NO��ԭ��֮һ��T��ʱ�����ݻ�Ϊ2 L���ܱ������г���10molN2��5molO2���ﵽƽ���NO�����ʵ���Ϊ2mol����T��ʱ�÷�Ӧ��ƽ�ⳣ��K= ��������������С�������λ���֣�
��1��������ȼ������ʱ����Ӧ��N2��g����O2��g�� 2NO��g�����ǵ�������β���к���NO��ԭ��֮һ��T��ʱ�����ݻ�Ϊ2 L���ܱ������г���10molN2��5molO2���ﵽƽ���NO�����ʵ���Ϊ2mol����T��ʱ�÷�Ӧ��ƽ�ⳣ��K= ��������������С�������λ���֣�
��2��һ������NO�����ֽ�Ĺ����У�NO��ת������ʱ��仯��������ͼ��ʾ������֪�� ��
��

 �ٷ�Ӧ2NO��g�� N2��g����O2��g��Ϊ������ȡ����ȣ� ��Ӧ��
�ٷ�Ӧ2NO��g�� N2��g����O2��g��Ϊ������ȡ����ȣ� ��Ӧ��
 ��һ���¶��£��ܹ�˵����Ӧ2NO��g�� N2��g����O2��g���Ѵﵽƽ����ǣ�����ţ� ��
��һ���¶��£��ܹ�˵����Ӧ2NO��g�� N2��g����O2��g���Ѵﵽƽ����ǣ�����ţ� ��
a�������ڵ�ѹǿ�������仯
b��NO��N2��O2��Ũ�ȱ��ֲ���
c��NO�ֽ�����ʺ�NO���ɵ��������
d����λʱ���ڷֽ�4mol NO��ͬʱ����2 mol N2
��3���ٵ�����������ϡ��ȼ��ʱ��β���е���Ҫ��Ⱦ��ΪNOx������CxHy����������ԭNO2���������������Ⱦ��
��֪��CH4(g)+4NO2(g) 4NO(g)+CO2(g)+2H2O(g) ��H1=-574kJ��mol-1
CH4(g)+4NO(g)=2N2(g)=CO2(g)+2H2O(g) ��H2
CH4(g)+2NO2(g) N2(g)+CO2(g)+2H2O(g) ��H3=-867kJ��mol-1
��H2= ��
��ʹ�ô������Խ�����β������Ҫ�к��ɷ�һ����̼��CO���͵������NOx��ת��Ϊ�����壬�÷�Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com