
�л����(C13H18O2)��һ�����ϣ���ϳ�·����ͼ��ʾ������A����Է�������ͨ���������Ϊ56�����ĺ˴Ź���������ʾֻ������壻D���Է���������Ӧ���ڴ�������������1 mol D��2 mol H2��Ӧ���������ң����к�������-CH3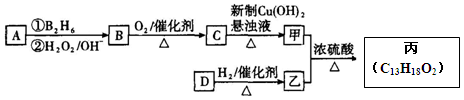
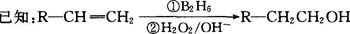
��1��A�Ľṹ��ʽΪ ���ҵķ���ʽΪ ��
��2��C������Cu(OH)2����Һ��Ӧ�Ļ�ѧ����ʽ_________________��
��3��D���������ŵ������� ��D�ж���ͬ���칹�壬��������������������ͬ��ͬ���칹���� �֣������������칹����
��4�������ҷ�Ӧ�Ļ�ѧ����ʽΪ ��
��5��д�����������������л���Ľṹ��ʽ �����һ�Ϊͬ���칹�壻����FeCl3��Һ����ɫ�����䱽���ϵ�һ�����ֻ�����֡�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
(12��)����ѧ�����л���ѧ������
ij�л�������K�ĺϳ�·�����£���
��֪��
III��E�ĺ˴Ź�������ͼ��ֻ��һ�����շ塣
��ش��������⣺
��1��A�ķ���ʽΪ_______��C��D�ķ�Ӧ����Ϊ_______��
��2������E��G���õ��Լ���NaOH��Һ�⣬����Ҫ���Լ���_______�����Լ����ƣ���
��3����������������C��ͬ���칹�干��_______�֡�
��������ˮ�����ӳɷ�Ӧ����ʹ�Ȼ�����Һ����ɫ
�۱�����������ȡ�������ܷ���������Ӧ
��4��J��һ�������¿��Է����ۺϷ�Ӧ�õ�һ�ָ߾���ø߾���Ľṹ��ʽΪ____ ____________________��
��5��D+J��K�Ļ�ѧ����ʽΪ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��15�֣�������F(ƥ����͡)���ڸߵ��̴�Ѫ֢�����ƣ���ϳ�·�����£�
��1��������D�й����ŵ�����Ϊ �� ��������
��2��A��B�ķ�Ӧ������ ��
��3��д��ͬʱ��������������A��һ��ͬ���칹��Ľṹ��ʽ
I�������к���������������������3�ֲ�ͬ��ѧ�������⣻����һO��Oһ��
��4��ʵ��D��E��ת���У�������X�ķ���ʽΪC19H15NFBr��д����ṹ��ʽ�� ��
��5����֪��������E��CF3COOH����������ת��Ϊ ����ת��ΪF������Ϊ�ϳ�·������Ʋ���ڵ�Ŀ���� ��
����ת��ΪF������Ϊ�ϳ�·������Ʋ���ڵ�Ŀ���� ��
��6�������ϳ�·���У�����۵IJ����D����� ���÷�Ӧԭ�����л��ϳ��о��й㷺Ӧ�á���д����
���÷�Ӧԭ�����л��ϳ��о��й㷺Ӧ�á���д���� Ϊ��Ҫԭ���Ʊ�
Ϊ��Ҫԭ���Ʊ� �ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
CH3CH2OH CH2��CH2
CH2��CH2 CH3CH3
CH3CH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
������(PETG)��������Ĺ���ȡ����ʺͿɻ��������õ��ص㣬�㷺Ӧ����ҽ����Ʒ���ճ�����Ʒ�ͻ�ױƷ��װ����ҵ��PETG�Ľṹ��ʽΪ��
PETG�²��ϵĺϳɺ�����·�����£�
�Իش��������⣺
��1��������IV�ķ���ʽΪ_______________________________��
��2��������I�Ľṹ��ʽ��______________��������II��һ��ͬ���칹��V���뱥��NaHCO3��Һ��Ӧ�ų�CO2�ҷ��ӽṹ�к���5����������V�Ľṹ��ʽΪ__________��
��3���ϳɵķ�Ӧ����Ϊ____________________________��
��4��������������ϩ��Br2ͨ�������ӳɺõ��IJ�����һ�������·���ȡ����Ӧ����ã���д������ȡ����Ӧ�Ļ�ѧ����ʽ��______________________________��
��5����һ�������£�CH3OH����̼����ϩ�� ��������PETG�����ķ�Ӧ�����в���֮һΪ̼�������[��ѧʽΪ(CH3O)2CO��һ�������Ļ���ԭ��]��д����Ӧ��ѧ����ʽ�����ñ귴Ӧ��������____________________��
��������PETG�����ķ�Ӧ�����в���֮һΪ̼�������[��ѧʽΪ(CH3O)2CO��һ�������Ļ���ԭ��]��д����Ӧ��ѧ����ʽ�����ñ귴Ӧ��������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
ú�����ɵõ���̿��ú���͡��ְ�ˮ�ͽ�¯������̿��ͨ������;����ȡ������ϩ�Ȼ�����Ʒ�����¿�ͼ��ʾ��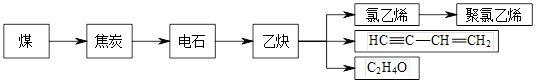
44.д���ɵ�ʯ��ȡ��Ȳ�Ļ�ѧ����ʽ________________________________��
45.������ϩ��Ʒ����ɰ�ɫ��Ⱦ��������ϩ�Ľṹ��ʽΪ_________________��
C2H4O���Ƿ���ȩ������________________�����Լ����ƣ������顣
46.��ú�����п��Է����һ����Ҫ��Һ̬����������д������Һ���������ڵ�����·�����Ӧ�Ļ�ѧ����ʽ__________________________________________________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��12�֣����ǻ���Ƥ���Ǻϳ��㾫����Ҫԭ�ϣ���Ϊ�ϳ����ǻ���Ƥ���·��֮һ
��֪��
�Իش��������⣺
��1��������II�Ľṹ��ʽΪ��
��2��������II��������III���л���Ӧ����
��3��������III��������Һ�з�����Ӧ��ѧ����ʽ
��4���л���XΪ������IV��ͬ���칹�壬��֪�л���X�������ص㣺���DZ��Ķ�λȡ���������NaHCO3��Ӧ�ų����壬���ܷ���������Ӧ����д��������X�Ľṹ��ʽ
��5������˵����ȷ���ǣ� ��
| A��������I���Ȼ�����Һ����ɫ | B��������II����NaHCO3��Һ��Ӧ |
| C��1mol������IV��ȫȼ������9��5molO2 | D��1mol������III����3 mol H2��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��16�֣�A��һ���л��ϳ��м��壬��ṹ��ʽΪ�� ��A�ĺϳ�·������ͼ������B��H�ֱ����һ���л��
��A�ĺϳ�·������ͼ������B��H�ֱ����һ���л��
��ش��������⣺
��1��A��̼ԭ�ӵ��ӻ����������_____��A�����ƣ�ϵͳ��������____���ڢಽ��Ӧ������___��
��2���ڢٲ���Ӧ�Ļ�ѧ����ʽ��________��
��3��C������CH2��C(CH3)COOH�����ʵ���֮��1:1��Ӧ������ᆳ�Ӿ۵õ����������۾��ľ�Ƭ����I��I�Ľṹ��ʽ��_______________��
��4���ڢ���Ӧ�Ļ�ѧ����ʽ��________________��
��5��д��������Ԫ������һ�ȴ���ֻ��2�֣������������칹����A��ͬ���칹��Ľṹ��ʽ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��������M�������ڵ��ƾ��в�ݮ�����ѡ�ӣ�ҡ�����������ζ��ʳ���㾫��M���ڷ����廯���������ֻ����һ��ֱ֧�����ܷ����Ӿ۷�Ӧ��ˮ�ⷴӦ�����M��Ħ������Ϊ162g��mol��1��ֻ��̼���⡢������ԭ�Ӹ���֮��Ϊ5:5:1��
(1)���������Ľṹ��ʽ��______________________��
(2)GΪ����������һ��ͬ���칹�壬����ӽṹģ������ͼ��ʾ(ͼ��������֮�����߱�ʾ������˫��)��G�Ľṹ��ʽΪ ��
(3)�÷�����AΪԭ�Ϻϳ�G��·�����£�
�ٻ�����E�еĹ�������________(������)��
��E�D��F�ķ�Ӧ������________��
F�D��G�Ļ�ѧ����ʽΪ__________________________________________________��
��д�����ַ�������������F���ȶ���ͬ���칹��Ľṹ��ʽ �� ��
��.�����ں��������ұ�����ֻ��һ��֧����
��.�ڴ��������£�1mol������������������ַ�Ӧ���������5mol H2��
��.�����ܷ���ˮ�ⷴӦ�������Է���������Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��6�֣�ijǿ������ҺX�к���Ba2����Al3����SiO32����NH4����Fe2����Fe3����CO32����SO42����NO3���е�һ�ֻ������ӣ�ȡ����Һ��������ʵ�飬��ʵ������ת����
����������Ϣ����ش��������⣺
��1����ҺX�г���H����Al3����NH4����SO42����϶������е������� ������ȷ���Ƿ��е������� ����Ҫȷ������ȷ�����������Ƿ���ڣ���ɿ������ǣ� ��
��2������E�Ļ�ѧʽΪ ��
��3����Ӧ�١��ڡ��ۡ����У�������������ԭ��Ӧ���� ������ţ�
��4��д�����������������A�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com