


 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡ��ɳ�и����ڶ����¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
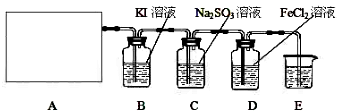
ijУ��ѧ��ȤС��Ϊ̽������Ũ����ķ�Ӧ,�����ͼ1��ͼ2��ʾװ�ý���ʵ�顣
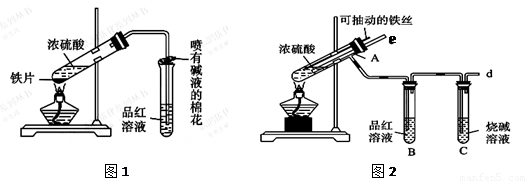
��1����˵����SO2���������ʵ�������� ��
��2��ͼ2�е�����e����Ҫ����Ϊ ��
��3������װ����ͼ2�е�NaOH��Һ������SO2β������ֹ��Ⱦ���罫�����Ϊ����KMnO4��Һ��ͬ�����ԴﵽĿ�ģ���д������KMnO4��Һ��SO2��Ӧ�Ļ�ѧ����ʽ��
��
��4���Ա�����ʵ��װ�ã����ѷ���ͼ2װ�ó����ܸ��õ������ж�����SO2��ֹ����Ⱦ�����⣬����һ���dz����Ե��ŵ㣬����Ϊ�� ��
��5����Ӧһ��ʱ���ֹͣ��Ӧ������ȴ���ý�ͷ�ι���ȡA�Թ��е���Һ���뵽����ˮ����Ϊ�����������������������ӵijɷ����������ֿ��ܣ�
��ֻ����Fe3+�� ��ֻ����Fe2+�� ����Fe3+����Fe2+��
Ϊȷ����Һ�ijɷ֣�ѡ�������Լ���
A��ϡHCl��Һ B��ϡ���� C��KSCN��Һ D������KMnO4��Һ
E��NaOH��Һ F��H2O2��Һ
�����������ص�ʵ��̽����
|
ʵ�鲽�� |
ʵ�������� |
|
1��ȡһ֧�ྻ���Թܣ��μ�1-2mL��������Һ�������Թ��еμӼ���KSCN��Һ |
��1�� ����˵��������� ��2�� ����˵����Һ�д���Fe3+������������ |
|
2��
|
�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com