A��B��C�����ֳ���������Ԫ�صĵ��ʣ�������DΪ��ɫҺ�壬E��һ�ֳ������������壮��ת����ϵ��ͼ����Ӧ�����Ͳ��ֲ�����ȥ����
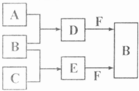
�Իش�
��1��E�ĵ���ʽ��
��
��2������X��B��D���ܷ�Ӧ���ɺ�ɫ���Թ���Y��Y�Ļ�ѧʽ��
Fe3O4
Fe3O4
��
��3������Z�����ڶԿ�������ɱ����������ˮ�����ʵȣ�Z��B�����Ԫ����ͬ��Z�����и�ԭ������������֮��Ϊ18��Z�����Ե⻯����Һ��Ӧ����B�͵ⵥ�ʣ���Ӧ�����ӷ���ʽ��
O3+2H++2I-=O2+I2+H2O
O3+2H++2I-=O2+I2+H2O
��
��4��ȡ0.3mol F������D��ֻ�Ϻ�������Һ����ͨ��0.2mol E��ַ�Ӧ�����õ���ˮ��Һ�и������ӵ�Ũ���ɴ�С��˳���ǣ�������H
+��
c��Na+����c��OH-����c��CO32-����c��HCO3-��
c��Na+����c��OH-����c��CO32-����c��HCO3-��
��
��5��E�Ĵ����ŷŻ������ܶ�����⣮�п�ѧ���������E��H
2�ϳ�CH
3OH��H
2O����E�����ۺ����ã�25�棬101kPaʱ���÷�Ӧ���Ȼ�ѧ����ʽ��
CO2��g��+3H2��g��=CH3OH��l��+H2O��1����H=-130.9kJ/mol
CO2��g��+3H2��g��=CH3OH��l��+H2O��1����H=-130.9kJ/mol
������֪�״���ȼ���ȡ�H=-726.5kJ?mol
-1��������ȼ���ȡ�H=-285.8kJ?mol
-1��

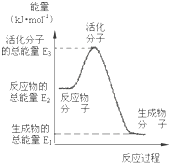 25�桢101kPaʱ�״����ӽ���Ĥȼ�ϵ�ؽ��״�����ת��Ϊ���������ַ�Ӧԭ���ǣ���CH3OH��g��+H2O��g��=CO2��g��+3H2��g����H1=+49.0kJ?mol-1
25�桢101kPaʱ�״����ӽ���Ĥȼ�ϵ�ؽ��״�����ת��Ϊ���������ַ�Ӧԭ���ǣ���CH3OH��g��+H2O��g��=CO2��g��+3H2��g����H1=+49.0kJ?mol-1

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�
 �Իش�
�Իش�
