
����Ŀ��X��Y��Z��W��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ��������Ϣ���±���
Ԫ�� | �����Ϣ |
X | X�Ļ�̬ԭ��L���������K���������2�� |
Y | Y�Ļ�̬ԭ�����������Ų�ʽΪ��nsnnpn+2 |
Z | Z����������Ϊ23��������Ϊ12�ĺ��� |
W | W�ж��ֻ��ϼۣ����ɫ���������ڿ����л�Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ |
��Wλ��Ԫ�����ڱ��� ���ڵ� �壬���̬ԭ��������� �����ӡ�
��X�ĵ縺�Ա�Y�� ��������������С������X��Y����̬�⻯���У����ȶ����� ��д��ѧʽ��
��д��Z2Y2��XY2��Ӧ�Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ�� ��
����Xԭ������ԭ���γɵĶ��ַ����У���Щ���ӵĺ˴Ź���������ʾ�������⣬д������һ�ַ��ӵ����ƣ� ����Ԫ�ء�X��Y��ԭ��Ҳ�ɹ�ͬ�γɶ��ַ��Ӻ�ij�ֳ����������ӣ�д������һ�ַ�������������ӷ�Ӧ�����ӷ���ʽ�� ��
���𰸡���4 �� 2 ��С H2O ��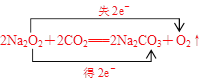
�����飨�����𰸺���Ҳ���֣� CH3COOH��HCO��3=CH3COO����H2O��CO2��
��������
��������Ϣ����֪X��Y��Z��W�ֱ�ΪC��O��n=2����Na������������=������+�������Ƶã���Fe���������������ɰ�ɫ��Ϊ����ɫ���Ϊ���ɫ������Ԫ�ء���Feλ�����ڱ���4���ڵ�����Ԫ�أ����̬ԭ�Ӽ۵����Ų�ʽΪ3d64s2���������2�����ӡ�������ͬһ���ڵ縺���������������ǽ���������ǿ֪X(C)�ĵ縺С��Y(O)��С��X����̬�⻯��CH4û��Y���⻯��H2O�ȶ�����H2O��H2O2�ȶ�����Na2O2��CO2��Ӧ�Ļ�ѧ����ʽΪ2Na2O2��2CO2=2Na2CO3��O2���ڱ����ת�Ƶķ������Ŀʱ��Ӧע��Na2O2����Ԫ�ػ��ϼ�Ϊ-1�ۡ�����С��Ϊ��ɢ�����⣬�𰸲�Ψһ���������к���������ԭ�ӵ����϶࣬����飨CH3CH2CH3���������飨CH3CH2CH2CH3������Ȳ��CH3C��CH���ȣ���C��H��O����Ԫ���γɵķ��Ӻܶ࣬���γɵ���������ֻ��HCO��3��������HCO��3��Ӧ�ķ��ӱ���Ϊ���ᣬ��CH3COOH�ȡ�
���㶨λ��������Ԫ���ƶ���Ϊ���п���ԭ�ӽṹ��Ԫ�����ڱ���Ԫ��������֪ʶ���˴Ź������ס�������ԭ��Ӧ�����ӷ���ʽ����д��ּ�ڿ��鿼����������ɡ��ṹ��������Ԫ�������ڱ��е�λ�õĹ�ϵ���ۺ�Ӧ������������ѧ���ķ�ɢ˼ά������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NaClO2��һ�ָ�Ч��ɱ����������Ҳ������Ư��֯��ȡ�������װ��̽��NaClO2���Ʊ���
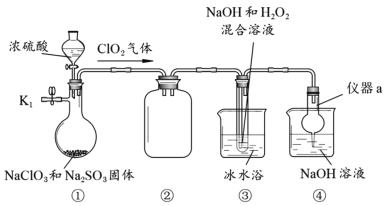
���������գ�
(1)����a������Ϊ__________��װ�âڵ�������________________��
(2)���װ�������Եķ�����________________________________________________��
(3)�ر�K1���ӷ�Һ©���м���һ����Ũ���ᣬװ�â�������NaClO2�Ļ�ѧ����ʽΪ2ClO2+2NaOH+H2O2��2NaClO2+2H2O+O2�����÷�Ӧ������������_____________��
(4)ʵ����ɺ�Ϊ��ֹװ���в������ж�������Ⱦ���������Խ��еIJ����ǣ���ֹˮ��K1��____________________________________________��
(5)��װ�â۵���Һ�л��NaClO2�������Ҫ�����м�ѹ����Ũ����________________������ϴ�ӡ�����ȡ�
(6)������NaClO2��3H2O����ʽ���ڣ���֪��NaClO2��3H2O![]() NaCl+O2��+3H2O����Ϊ�ⶨ���ù�����NaClO2��3H2O���������������ռ��������ľ����������ʵ�飺ȡ��Ʒ����Ϊa g�����պ��غõ�����b g��������NaClO2��3H2O������������__________��
NaCl+O2��+3H2O����Ϊ�ⶨ���ù�����NaClO2��3H2O���������������ռ��������ľ����������ʵ�飺ȡ��Ʒ����Ϊa g�����պ��غõ�����b g��������NaClO2��3H2O������������__________��
����������Ӧ4[NaClO2��3H2O]![]() 2NaCl+2NaClO3+O2��+12H2O������ʵ���õ�ֵ��__________����ѡ����ƫ��������ƫС��������������
2NaCl+2NaClO3+O2��+12H2O������ʵ���õ�ֵ��__________����ѡ����ƫ��������ƫС��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����֪���������Ȼ�ѧ����ʽ��
C3H8��g��+5O2��g�� 3CO2��g��+4H2O��l�� ��H=��2220.0 kJ��mol-1
H2O��l�� H2O��g�� ��H=+44.0 kJ��mol-1
��0.5 mol����ȼ������CO2����̬ˮʱ�ͷŵ�����Ϊ ��
��2����֪��TiO2��s����2Cl2��g�� TiCl4��l����O2��g�� ��H����140 kJ��mol��1
2C��s����O2��g�� 2CO��g�� ��H����221 kJ��mol��1
д��TiO2�ͽ�̿��������Ӧ����TiCl4��CO������Ȼ�ѧ����ʽ��
��3����ѧ���ѻ���˼��������о������N4���ӣ���ṹΪ�������壨����ͼ��ʾ ��������������ơ���֪����1molN��N������193kJ����������1molN
��������������ơ���֪����1molN��N������193kJ����������1molN![]() N������941kJ��������1molN4����ת��Ϊ2molN2ʱҪ�ų�______________kJ������
N������941kJ��������1molN4����ת��Ϊ2molN2ʱҪ�ų�______________kJ������
��4������������ɴ���ʹ�õ�������ȼ�ϵ�أ������ܷ�ӦΪ��
2H2+O2 2H2O���������ҺΪϡH2SO4��Һ����طŵ�ʱ�ǽ�___________��ת��Ϊ__________�ܡ���缫��Ӧʽ�ֱ�Ϊ������_________________������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£����и���������ָ����Һ���ܴ����������
A. ��ɫ����Һ:A13+��NH4+��Cl-��S2-
B. �ں�0.1 mol/LHClO��Һ��:Cu2+��Fe2+��Cl-��SO42-
C. ![]() =1��10-13mol��L-1����Һ:Na+��K+��SiO32-��CO32-
=1��10-13mol��L-1����Һ:Na+��K+��SiO32-��CO32-
D. �ں�0. 1mol/LFeCl3��Һ��:K+��NH4+��I-��SCN-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)ʵ�����ý���ͭ��ϡ������ȡNO�����ӷ���ʽΪ_________________________��
(2)NO���ж����壬ijѧ��Ϊ��ֹ��Ⱦ���÷�Һ©�����ձ�װ����һ���ġ����濪���á������ͣ��NO���巢��װ�ã���ͼ����ʾ��

��ʵ������û��ͭ˿����ֻ��Сͭ������ʹ������װ�ý���ʵ��ʱ������˿״���ϰ���ͭ���Դ���ͭ˿����ʵ�飬����˿״���ϵijɷֿ�����________(����ĸ)��
A���� B���� C���� D������
�ڴ�Һ©���Ļ���ʹ��Ӧ���У��ڷ�Һ©����ʵ�ʿ����������Ǻ���ɫ�ģ�ԭ����______________(�ѧ����ʽ)��
(3)Ϊ֤��ͭ˿��ϡ���ᷴӦ���ɵ�ȷʵ��NO��ijѧ���������һ����ͼ����ʾ��װ����ȡNO����Ӧ��ʼ������U�ι��Ҷ˹۲쵽��ɫ��NO���塣
�ٳ������ܵ�������__________________________________________________��
���÷�Ӧֹͣ�IJ���������ԭ����_______________________________________________��
(4)�����ռ�NO�����װ�ã���������________(����ĸ)��
a. b.
b.![]() c.
c. d.
d.![]() e.
e.![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ����ʵ��ʱ������þʧ�����������������̼��������ѻ�����ȴ��ʵ����ʦ��ʱ��ֹ��ԭ����CO2����֧��þȼ�շ������·�Ӧ��2Mg+CO2![]() 2MgO+C�����й��ڸ÷�Ӧ���ж���ȷ����
2MgO+C�����й��ڸ÷�Ӧ���ж���ȷ����
A. MgԪ�ػ��ϼ���0�����ߵ�+2�ۣ�����MgO�ǻ�ԭ����
B. �ɴ˷�Ӧ�����ж�������CO2>MgO����ԭ��Mg>C
C. CO2�������������������ԣ�����������Ӧ
D. Mgԭ��ʧȥ�ĵ�����Ŀ����Oԭ�ӵõ��ĵ�����Ŀ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ��ͨ������SiO2 ��̼��Ӧ����ȡ�裬д����Ӧ�Ļ�ѧ����ʽ___________________��
��ҵ�ϻ���������þ��ȡ�裬��ӦΪ2Mg+SiO2 = 2MgO+Si��ͬʱ�ᷢ������Ӧ��2Mg + Si = Mg2Si����ͼ�ǽ���Mg��SiO2��Ӧ��ʵ��װ�ã��Իش��������⣺

��1������O2��H2O��g���Ĵ��ڶԸ�ʵ���нϴ�Ӱ�죬ʵ����Ӧͨ������X��Ϊ���������Թ��еĹ���ҩƷ��ѡ��________(�����)��
a��ʯ��ʯ������b��п��������c������
��2��ʵ�鿪ʼʱ��������ͨһ��ʱ��X���壬�ټ��ȷ�Ӧ��������� ___________________________������Ӧ���������߾ƾ��ƣ���Ӧ�ܼ������У���ԭ����______________________��
��3����Ӧ��������ȴ������ʱ������Ӧ��Ļ�����м���ϡ���ᣬ�ɹ۲쵽�����Ļ��ǣ������������ԭ���Ǹ�����Mg2Si������Ѹ�ٷ�Ӧ����SiH4�����飩���壬Ȼ��SiH4��ȼ���û�ѧ����ʽ��ʾ��������Ӧ��________________________��___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵���д������( )
A.![]() ��������ԭ���ϵŵ��Ӷ�����1�����幹��Ϊƽ��������
��������ԭ���ϵŵ��Ӷ�����1�����幹��Ϊƽ��������
B.Ԫ�����ڱ�������������Ԫ���У������Ӱ뾶��С����![]()
C.���ʯת��ΪʯīΪ���ȷ�Ӧ��˵����ͬ������ʯī�Ƚ��ʯ�ȶ�
D.![]() ��
��![]() ���ǷǼ��Է���
���ǷǼ��Է���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ҹ�����ij�κ��ġ�����һ��Na2CO3��NaCl�Ļ�����ͨ�����·����Կ����Ʒ����ɺͺ������з����ⶨ��
��.���ԲⶨNaCl�Ĵ�����
(1)ȡ��Ʒ��������ˮ����������ϡ�����ַ�Ӧ���ٵμ�_________________��Һ���ܹ۲쵽��_______________________������˵������к���NaCl��
��.�����ⶨNa2CO3�ĺ�����
ȷ����w g��Ʒ��������װ���У������м�������ϡ���ᣬͨ���ⶨ������������������Na2CO3�ĺ�������ش�����������⣺

(2)�ⶨװ���У�����a��������___________________________________________��
(3)Ϊ��߲ⶨ��ȷ�ȣ���Һb����ѡ��___________________________________��
�ٱ���ʳ��ˮ �ڱ���NaHCO3��Һ �۳���ʯ��ˮ ������KMnO4��Һ
(4)��������ȡ��Ʒ���������Ʒ�Ӧʱ������������������30mL����,���������ǰ������װ�ö���ʱ��װ���ڵ�װҺ����Һ�������Ϊ��������_______________________(�����߿��ж�Ӧ�����ĸ���)��
(5)����������ѡ������Ϊ�����Ķ���Ϊ��㣬���յ����Ϊ17.60mL����Ӧ������������IJⶨ���Ϊ_____________mL����ʵ���¶��µ�����Ħ�����ΪVmL.mol-1���ɴ˿ɵó���Ʒ��Na2CO3�ĺ�������ʽΪ�� __________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com