
����Ŀ��NaClO2��һ�ָ�Ч��ɱ����������Ҳ������Ư��֯��ȡ�������װ��̽��NaClO2���Ʊ���
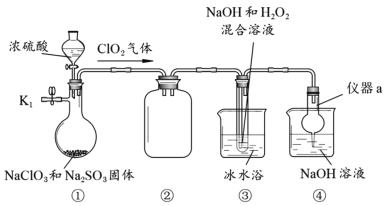
���������գ�
(1)����a������Ϊ__________��װ�âڵ�������________________��
(2)���װ�������Եķ�����________________________________________________��
(3)�ر�K1���ӷ�Һ©���м���һ����Ũ���ᣬװ�â�������NaClO2�Ļ�ѧ����ʽΪ2ClO2+2NaOH+H2O2��2NaClO2+2H2O+O2�����÷�Ӧ������������_____________��
(4)ʵ����ɺ�Ϊ��ֹװ���в������ж�������Ⱦ���������Խ��еIJ����ǣ���ֹˮ��K1��____________________________________________��
(5)��װ�â۵���Һ�л��NaClO2�������Ҫ�����м�ѹ����Ũ����________________������ϴ�ӡ�����ȡ�
(6)������NaClO2��3H2O����ʽ���ڣ���֪��NaClO2��3H2O![]() NaCl+O2��+3H2O����Ϊ�ⶨ���ù�����NaClO2��3H2O���������������ռ��������ľ����������ʵ�飺ȡ��Ʒ����Ϊa g�����պ��غõ�����b g��������NaClO2��3H2O������������__________��
NaCl+O2��+3H2O����Ϊ�ⶨ���ù�����NaClO2��3H2O���������������ռ��������ľ����������ʵ�飺ȡ��Ʒ����Ϊa g�����պ��غõ�����b g��������NaClO2��3H2O������������__________��
����������Ӧ4[NaClO2��3H2O]![]() 2NaCl+2NaClO3+O2��+12H2O������ʵ���õ�ֵ��__________����ѡ����ƫ��������ƫС��������������
2NaCl+2NaClO3+O2��+12H2O������ʵ���õ�ֵ��__________����ѡ����ƫ��������ƫС��������������
���𰸡������ ����ȫƿ����ֹ������Һ���������� �ر�K1���رշ�Һ©���������ڢܵ��ձ��м�ˮ��û������¶ˣ���������ƿ�ȣ������г������ݣ�ֹͣ���ȣ����и�����¶���һ��ˮ����һ��ʱ�䲻�䣬������������ O2 ��K1��ͨ����������������������� ���½ᾧ ![]() ��
��![]() ƫС
ƫС
��������
��ʵ��װ��ͼ��֪��װ�âٵ������������Ի����£������ƺ��������Ʒ���������ԭ��Ӧ�Ʊ�ClO2���壻װ�âڵ�����������ȫƿ����ֹ������Һ���������У�װ�â۵��������ڼ��������£�ClO2������������ⷢ��������ԭ��Ӧ�Ʊ�NaClO2��װ�âܵ����������ն����ClO2���壬��ֹ��Ⱦ������
��1������a������Ϊ����ܣ�װ�âڵ�����������ȫƿ����ֹ������Һ���������У��ʴ�Ϊ������ܣ���ȫƿ����ֹ������Һ���������У�
(2)���װ������������Ҫ�γ��ܱ�ϵͳ��Ȼ��ͨ�������¶��γ�ѹǿ���������ǹر�K1���رշ�Һ©���������ڢܵ��ձ��м�ˮ��û������¶ˣ���������ƿ�ȣ������г������ݣ�ֹͣ���ȣ����и�����¶���һ��ˮ����һ��ʱ�䲻�䣬�����������ã��ʴ�Ϊ���ر�K1���رշ�Һ©���������ڢܵ��ձ��м�ˮ��û������¶ˣ���������ƿ�ȣ������г������ݣ�ֹͣ���ȣ����и�����¶���һ��ˮ����һ��ʱ�䲻�䣬�����������ã�
��3���������ѧ����ʽ��֪����Ӧ�й�����������Ԫ�ػ��ϼ����߱�����������������Ӧ�����������Ϊ��ԭ��������Ϊ��������ʴ�Ϊ��O2��
��4��ʵ����ɺ�Ϊ��ֹװ���в�����ClO2������Ⱦ������Ӧ��ֹˮ��K1����K1��ͨ���������������ClO2���������У�������������Һ���գ���ֹ��Ⱦ�������ʴ�Ϊ����K1��ͨ����������������������У�
��5������Һ����ȡ���壬һ���������Ũ�������½ᾧ�����ˡ�ϴ�ӡ�����ķ��������װ�â۵���Һ�л��NaClO2�������Ҫ�����м�ѹ����Ũ�������½ᾧ�����ˡ�ϴ�ӡ�����ʴ�Ϊ�����½ᾧ��
��6���������֪��NaClO2��3H2O���������صõ�NaCl��������ٵ�����Ϊ������ˮ����������������ˮ������Ϊ��a��b���������������ʵ���Ϊxmol���ɷ���ʽ��֪ˮ�����ʵ���Ϊ3xmol����ɵ�32x+18��3x=��a��b�������x=![]() ���ɷ���ʽ�ɵù�ϵʽNaClO2��3H2O��O2������n��NaClO2��3H2O��= n��O2��=
���ɷ���ʽ�ɵù�ϵʽNaClO2��3H2O��O2������n��NaClO2��3H2O��= n��O2��=![]() mol��������NaClO2��3H2O����������Ϊ
mol��������NaClO2��3H2O����������Ϊ![]() =
=![]() ��
��![]() ������������Ӧ4[NaClO2��3H2O]
������������Ӧ4[NaClO2��3H2O]![]() 2NaCl+2NaClO3+O2��+12H2O�����ᵼ�����պ��غ������������b��������
2NaCl+2NaClO3+O2��+12H2O�����ᵼ�����պ��غ������������b��������![]() ��С���ʴ�Ϊ��
��С���ʴ�Ϊ��![]() ��
��![]() ��ƫС��
��ƫС��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ʒӵ�һ�ֺϳ�·�����£�

������������ȷ����
A. ��NaOHˮ��Һ�м��ȣ�������X�ɷ�����ȥ��Ӧ
B. ��һ�������£�1mol������Y������4molBr2
C. ��FeCl3��Һ�ɼ�����X��Y
D. ��һ��������������Y����HCHO�������۷�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ݱ������ҹ���ѧ�����Ƴ���ʯīϩΪ����Ĵ�������25������H2O2ֱ�ӽ�CH4ת��Ϊ�����л������Ҫԭ����ͼ��ʾ��

����˵������ȷ����( )
A.ͼ��![]() ����H2O2
����H2O2
B.����i��ii���ܷ�Ӧ����ʽ��![]()
C.����ͼ��֪������iv���ɵ�H2O�����е�Hԭ��ȫ������H2O2
D.��������ԭ�����Ʋⲽ��vi����HCOOH��H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���̷��Ǻ���һ�����ᾧˮ����������,�ڹ�ũҵ�����о�����Ҫ����;��ijͬѧ������������ȡ������������Ҫ�ɷ�ΪFe2O3��SiO2��Al2O3���������������ʣ���ȡ��ˮ������������FeSO4��7H2O�����������ͼ���̣�

��֪��ijЩ���������ӿ���ͨ��������pH��[��������Һ������ԣ�pH=-lgc(H��), pHֵԽ����Խǿ]ʹ��ת��Ϊ����������2Ϊ��ɫ������
��1����������֮���ʵ�������______________________��
��2���ܽ�����ѡ�õ��������ܷ������ᣬ��˵������___________________________��
��3����֤��Һ2���Ƿ���Fe3+�ķ�����_______________________________________��
��4���Լ�X��������_________________��
��5������Һ2�еõ��̷��IJ�������Ϊ��______________����ȴ�ᾧ�����ˡ���Ȼ�������һϵ�в�����û���õ���������________________�����˿�����ţ�
A.������ B.ʯ���� C.�ձ� D.������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CH4��Cl2����CH3Cl�ķ�Ӧ���̣��м�̬���ʵ�������ϵ����ͼ���й�˵���������
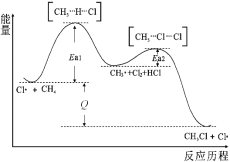
A.Cl������Cl2�ڹ����»�ѧ���������ɵģ��ù��̿ɱ�ʾΪ��![]()
B.��Ӧ����һ����CH3CH3������
C.ͼ�еķ�Ӧ��Q>0�����С��Ea1��Ea2��
D.CH4ת��ΪCH3Cl�Ĺ��̣�C��H�������˶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���й����պ�����ҵ���ٷ�չ����ͭ�Ͻ�㷺���ں��չ�ҵ������ͭ�Ͻ��и�����л��������Ʊ�ͭ������Ʒ�Ĺ������£�

ע��A1(OH)3��Cu(OH)2�ֽ��¶ȷֱ�Ϊ450���80��
(1)�ڵ�⾫����ʱ����������Ϊ___________��
(2)�ӿ�����(��������)����ϡH2SO4���ʵĴ�ʩΪ___________(д������)��
(3)����A��ϡHNO3��Ӧ�������������ڿ�����Ѹ�ٱ�Ϊ����ɫ������A��ϡHNO3��Ӧ�����ӷ���ʽΪ______________________��
(4)���˲�����Ҫ�IJ����������ձ����___________��
(5)��������B�����Ϊ___________�������ɹ���B�Ĺ����У������NaOH�ļ���������NaOH�����������������ķ�Ӧ�����ӷ���ʽΪ___________��
(6)���ս�ͨ����������ԭ��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.��5.1gþ���Ͻ�ķ�ĩ��������������������Һ�У��õ�2.24LH2����״���²ⶨ����
��1���Ͻ���þ������Ϊ________��
��2��д���úϽ���������������������Һ�Ļ�ѧ����ʽ��_________________________��
��. ��AlCl3��MgCl2�Ļ����Һ����μ�������������Һ�����ɳ��������ʵ���������������Ƶ����ʵ����Ĺ�ϵ��ͼ��ʾ��
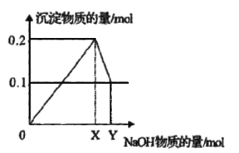
��3���ڻ����Һ��AlCl3��MgCl2�����ʵ���֮��Ϊ_____________��
��4��д��XY�η�����Ӧ�����ӷ���ʽ_____________________________��
��5��X=____________��Y=____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��![]() ��ˮ������EΪ���������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�
��ˮ������EΪ���������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�
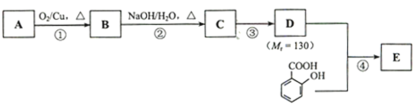
��ش��������⣺
��1��һԪ��A��������������ԼΪ21.6%����A�ķ���ʽΪ______���˴Ź�����������ʾ��A�������ֲ�ͬ��ѧ�������⣬�ҷ����֮��Ϊ3��2��2��2��1��A������Ϊ______��
��2��B����������Һ������Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ______��
��3��C��____�ֽṹ����һ��ȡ��������C�����������ţ���ʹ�õ��Ⱥ�˳��д�������Լ���___��
��4���ڢ۲��ķ�Ӧ����Ϊ______��D���������ŵ�����Ϊ_____��
��5��д��ͬʱ��������������ˮ��������ͬ���칹��Ľṹ��ʽ��______��
a����������6��̼ԭ����һ��ֱ���ϣ�
b�����������������Ű���ˮ������еĹ����ţ�
��6���ڢܲ��ķ�Ӧ����Ϊ______��д��E�Ľṹ��ʽ��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��W��Ԫ�����ڱ�ǰ�������еij���Ԫ�أ��������Ϣ���±���
Ԫ�� | �����Ϣ |
X | X�Ļ�̬ԭ��L���������K���������2�� |
Y | Y�Ļ�̬ԭ�����������Ų�ʽΪ��nsnnpn+2 |
Z | Z����������Ϊ23��������Ϊ12�ĺ��� |
W | W�ж��ֻ��ϼۣ����ɫ���������ڿ����л�Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ |
��Wλ��Ԫ�����ڱ��� ���ڵ� �壬���̬ԭ��������� �����ӡ�
��X�ĵ縺�Ա�Y�� ��������������С������X��Y����̬�⻯���У����ȶ����� ��д��ѧʽ��
��д��Z2Y2��XY2��Ӧ�Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ�� ��
����Xԭ������ԭ���γɵĶ��ַ����У���Щ���ӵĺ˴Ź���������ʾ�������⣬д������һ�ַ��ӵ����ƣ� ����Ԫ�ء�X��Y��ԭ��Ҳ�ɹ�ͬ�γɶ��ַ��Ӻ�ij�ֳ����������ӣ�д������һ�ַ�������������ӷ�Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com