
X��Y��Z��������Ԫ�صĵ����ڳ����¶��dz�������ɫ���壬���ʵ�����������֮���������������Ӧ���ɷֱ���˫�ˡ����˺��ĺ˵ļס��ҡ������ַ��ӣ����ҡ��������к���XԪ�ص�ԭ�Ӹ�����Ϊ2��3����ش��������⣺
(1)Ԫ��X��������________��
(2)������Y�����ڳ����»�Ͼ�������������Ļ�ѧʽΪ________������һ��������ת��Ϊ���ҵķ�Ӧ����ʽΪ_______________________________________��
(3)�ٻ����ﶡ��X��Y��Z����Ԫ�أ�0.1 mol·L��1����Һ��pHΪ1��������������ʵ���֮��Ϊ1��1��Ϻ�����������ľ���ṹ�к��еĻ�ѧ��Ϊ________(ѡ�����)��
a��ֻ�����ۼ� b��ֻ�����Ӽ�
c���Ⱥ����Ӽ����ֺ����ۼ�
�ڳ����£����ˮ��Һ��pH________7(���������������)����ԭ����________________________________________________________________________
(�����ӷ���ʽ��ʾ)��
 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��«����������ϩ���ӻ�������п�������������Ԥ����Ѫ�ܼ��������á�ij��������������ºϳ�·�ߣ�
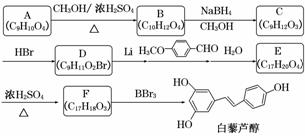
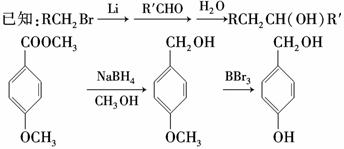
����������Ϣ�ش��������⣺
(1)��«���ķ���ʽ��____________��
(2)C��D�ķ�Ӧ������____________��E��F�ķ�Ӧ������____________��
(3)������ A ���� FeCl3 ��Һ������ɫ��Ӧ������ NaHCO3��Ӧ�ų� CO2���Ʋ���˴Ź�������(1H��NMR)����ʾ��________�ֲ�ͬ��ѧ��������ԭ�ӣ��������Ϊ________��
(4)д��A��B ��Ӧ�Ļ�ѧ����ʽ��______________________________________��
(5)д�������� D��E �Ľṹ��ʽ��D________________________________________��
E__________________��
(6)������ �ж���ͬ���칹�壬д��������������������ͬ���칹��Ľṹ��ʽ��
�ж���ͬ���칹�壬д��������������������ͬ���칹��Ľṹ��ʽ��
________________________________________________________________________��
���ܷ���������Ӧ���ں������ұ�����ֻ�����ֲ�ͬ��ѧ��������ԭ�ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)BF3��һ����ˮ�γ�(H2O)2·BF3����Q��Q��һ�������¿�ת��ΪR��

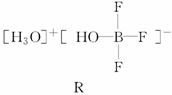
�پ���Q�и�����������������漰________(�����)��
a�����Ӽ���b�����ۼ���c����λ����d����������e�������f�����»���
��R�������ӵĿռ乹��Ϊ____________�������ӵ�����ԭ�ӹ������________�ӻ���
(2)��֪����( )���������ԣ���Ka��1.1��10��10��ˮ�����һ�������γɵ�����
)���������ԣ���Ka��1.1��10��10��ˮ�����һ�������γɵ����� ���γɷ�����������ݴ��жϣ���ͬ�¶��µ���ƽ�ⳣ��Ka2(ˮ����)________Ka(����)(���������)����ԭ����_____________________________________________��
���γɷ�����������ݴ��жϣ���ͬ�¶��µ���ƽ�ⳣ��Ka2(ˮ����)________Ka(����)(���������)����ԭ����_____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
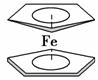 ��ï������[Fe(C5H5)2]��һ�ֽ����л�������ȼ���͵����Ӽ����������ȼ�յ�Ч�ʺ�ȥ�̣�����Ϊ���������ǵ�Ϳ�ϵȡ����Ľṹ����ͼ��ʾ������˵����ȷ����
��ï������[Fe(C5H5)2]��һ�ֽ����л�������ȼ���͵����Ӽ����������ȼ�յ�Ч�ʺ�ȥ�̣�����Ϊ���������ǵ�Ϳ�ϵȡ����Ľṹ����ͼ��ʾ������˵����ȷ����
A����ï����Fe2���뻷���ϩ����(C5H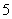 )֮��Ϊ���Ӽ�
)֮��Ϊ���Ӽ�
B��FeԪ�������ڱ��е�λ���ǵ������ڢ�A
C��Fe2���ĵ����Ų�ʽΪ1s22s22p63s23p63d6
D�������ϩ( )�е�̼ԭ����sp�ӻ���sp2�ӻ���sp3�ӻ�
)�е�̼ԭ����sp�ӻ���sp2�ӻ���sp3�ӻ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������H��He��N��Na��Mg��Si��Ԫ�أ�������δ������Դ���⡣
(1)3He�Ǹ�Ч����ԭ�ϣ���ԭ�Ӻ���������Ϊ________��
(2)Na��ԭ�ӽṹʾ��ͼΪ________��Na����������ȫȼ�����ò���ĵ���ʽΪ________��
(3)MgCl�ڹ�ҵ��Ӧ�ù㷺������MgO�Ʊ���
��MgO���۵��BaO���۵�________(��ߡ��͡�)��
��������ij��ʯ�������õ���MgO�к�������SiO2����ȥSiO2�����ӷ���ʽΪ__________________________��SiO2�ľ�������Ϊ________��
��MgO��̿�ۺ�������һ�������·�Ӧ���Ʊ�MgCl2����β����������NaOH��Һ��ȫ���գ������ɵ���Ϊ________________(д��ѧʽ)��
(4)�����к��зḻ��3He��������������1 kg 3He��ͬʱ�ɵ�6000 kg H2��700 kg N2����������H2��N2Ϊԭ�Ͼ�һϵ�з�Ӧ��������̼�����________kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��Ϊ������Ԫ�أ�X��Y����ͬһ���ڣ�X��Z����ͼ����ӷֱ�ΪX2����Z����Y����Z��������ͬ�ĵ��Ӳ�ṹ������˵����ȷ����(����)
A��ԭ��������������X>Y>Z
B�����ʷе㣺X>Y>Z
C�����Ӱ뾶��X2��>Y��>Z��
D��ԭ��������X>Y>Z
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
230Th��232Th���ʵ�����ͬλ�أ�232Th����ת����233U�������й�Th��˵����ȷ����
A. Th Ԫ�ص���������232 B. Th Ԫ�ص����ԭ��������231
C. 232Th ת����233U�ǻ�ѧ�仯 D. 230Th��232Th�Ļ�ѧ������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪
��1��H2O(g) == H2O(l) ��H1��-Q1 kJ��mol-1
��2��CH3OH(g) == CH3OH(l) ��H2��-Q2 kJ��mol-1
��3��2CH3OH(g) +3O2(g) == 2CO2(g)��4H2O(g) ��H3��-Q3 kJ��mol-1
��Q1��Q2��Q3������0����Ҫʹ32gҺ̬�״���ȫȼ�գ����ָ������£��ų�������Ϊ����λ��kJ�� �� ��
A��Q1+Q2+Q3 B�� 0.5 Q3-Q2+2Q1 C�� 0.5 Q3+ Q2-2Q1 D�� 0.5��Q1+Q2+Q3��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com