
| ���� | �ṹ��ʽ | ˮ���ܽ��/g��25�棩 | �۵�/�� | �е�/�� |
| X | 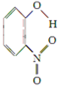 | 0.2 | 45 | 100 |
| Y | 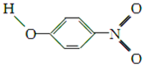 | 1.7 | 114 | 295 |
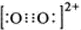 ����1��O22+����2���м�����1 mol O22+�У�����2NA�� �м����ʴ�Ϊ��
����1��O22+����2���м�����1 mol O22+�У�����2NA�� �м����ʴ�Ϊ��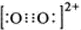 ��2NA��
��2NA��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��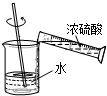 ϡ��Ũ���� |
B��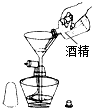 ���Ӿƾ� |
C��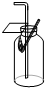 �������� |
D��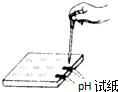 ����ҺpH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����߶���ˮ�⣬ˮ������ղ��ﲻͬ |
| B������C��H��O����Ԫ�ص�����������ͬ���һ�Ϊͬ���칹�� |
| C�����Ƕ������࣬�Ҷ��Ǹ߷��ӻ����� |
| D�������ã�C6H10O5��n��ʾ���������ܷ���������Ӧ������ά�ز��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��NH4Cl |
| B��KAlO2 |
| C��KHCO3 |
| D��NaHSO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ú�ˮ���˷����ǽ�����ת��Ϊ���� |
| B���Ӻ�ˮ����ȡ�嵥��һ�����л�ѧ�仯 |
| C����ˮ�е���Ԫ����UCl4����ʽ���ڣ��������������ӽ�����֬�ɵõ��˵��� |
| D�������з���ˮֱ����ȴ�������ɼ���ú̿��ʹ�����������ڻ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ũ���� | B��ϡ���� |
| C��Ũ���� | D��Ũ����������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��H2S��NH3���Ǽ۵�������Ϊ8�ļ��Է��� |
| B��HS-��HCl���Ǻ�һ�����Լ���18�������� |
| C��1molD216O�к����ӡ����ӡ����Ӹ�10NA��NA���������ӵ�������ֵ�� |
| D��CH2C12��CCl4���������幹�͵ķǼ��Է��� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com