
| A����ּ�����Ʒ���ü�ʯ�������ݳ����壬����b g |
| B����������Ʒ�������ٷ����仯���Ƶ�������Ϊb g |
| C������Ʒ�м���������ϡ���ᣬ����ˮ���ռ��ݳ����壬��b mL���� |
| D������Ʒ�м���������ϡ���ᣬ��ַ�Ӧ�������ɵ�����ȫ��ͨ�뵽����Ba��OH��2��Һ�У����ˡ�ϴ�ӡ���ɣ���b g���� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
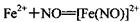 ����ɫ)����֪��ͬŨ�ȵ����������Ա�Fe3+��ǿ��
����ɫ)����֪��ͬŨ�ȵ����������Ա�Fe3+��ǿ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
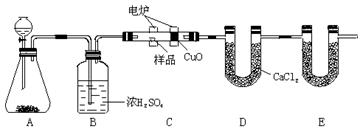
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ���� | ʵ �� | ʵ��Ŀ�� |
| A | Cl2��Br2�ֱ���H2��Ӧ | �Ƚ�������ķǽ�����ǿ�� |
| B | ͬ���²ⶨ��ͬŨ�ȵ�Na2CO3�� ��Na2SO4��Һ������� | �Ƚ�������̼�������ǿ�� |
| C | AlCl3��MgCl2��Һ��ͨ�˹������� | �Ƚ�þ�������ʵĻ�ԭ��ǿ�� |
| D | ͬ������ͬһ��·�ֱ�ⶨͬŨ�ȵ� �����ijһԪ����Һ�������� | �Ƚϸ�һԪ�����������Ե�ǿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NO2ͨ��FeSO4��Һ�� |
| B��CO2ͨ��CaCl2��Һ�� |
| C��NH3ͨ��AlCl3��Һ�� |
| D��SO2ͨ�����ữ��Ba(NO3)2��Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| A������ | B��BaCl2��Һ | C������ | D��Na2CO3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
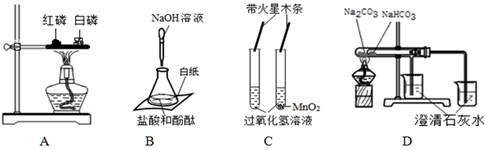
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
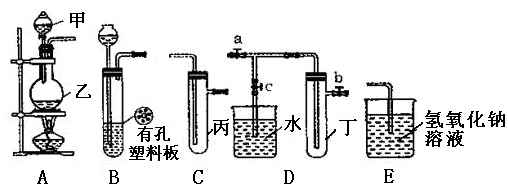
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com