
����Ŀ��A��B��C��D��E��FΪ����������Ԫ�أ���ԭ��������������A��B ��Ԫ��������ۺ������֮��Ϊ�㡣E��Aͬ���壻D��F�ǵؿ��к�����ߵķǽ���Ԫ�غͽ���Ԫ�ء�
(1)д��CԪ�ص�Ԫ������_______���������ڱ��е�λ��__________________��E���ӽṹʾ��ͼΪ__________________��E2D2�ĵ���ʽ________________________��
(2)��A��C��ԭ�Ӹ�����Ϊ5�U1��ɵ�һ�ֻ�������и�ԭ�Ӿ��ﵽ�ȶ��ṹ���û�������������ѧ������Ϊ_________________________��
(3)E2D2��A2D��Ӧ�����ӷ���ʽΪ__________________________________________��
(4)��C��E��ɵ�һ�����ӻ����ﻯѧʽΪE3C���û��������������ӵİ뾶�ɴ�С��˳����____________(��Ԫ�ط��ű�ʾ)���û�������ˮ���ҷ�Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ___________________________________��
���𰸡��� ��2���ڵ�VA�� 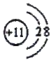
![]() ���Ӽ������ۼ� 2Na2O2+2H2O=4Na++4OH�C+O2�� N3�C>Na+ Na3N+3H2O=3NaOH+NH3��
���Ӽ������ۼ� 2Na2O2+2H2O=4Na++4OH�C+O2�� N3�C>Na+ Na3N+3H2O=3NaOH+NH3��
��������
���������Ϣ������Ԫ�ص����࣬���ݺ�������Ų�����ж�Ԫ�������ڱ��е�λ�ã�����Ԫ�����ʼ����ʵ���ɣ���д���ӽṹʾ��ͼ��������ʵĵ���ʽ���жϻ��ϼ����ͼ���д��ط�Ӧ����ʽ��
A��B��C��D��E��FΪ����������Ԫ�أ���ԭ��������������A��B ��Ԫ��������ۺ������֮��Ϊ�㣬��AΪH��BΪC��E��Aͬ���壬��EΪNa��D��F�ǵؿ��к�����ߵķǽ���Ԫ�غͽ���Ԫ�أ���DΪO��FΪAl��CΪN��
(1)CԪ��λ��C��O֮�䣬����C��Ԫ������Ϊ�����������ڱ��е�λ��Ϊ��2���ڵ�VA�壻EΪNa�������ӽṹʾ��ͼΪ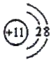 ��E2D2�ǹ������ƣ�����ʽΪ
��E2D2�ǹ������ƣ�����ʽΪ![]() ��
��
�ʴ�Ϊ��������2���ڵ�VA�壻 ��
��![]() ��
��
(2)���������Ϣ֪��A��C��ɵĻ�����Ϊ笠�����(NH4+)���⸺����(H-)��ɵ����ӻ������⻯泥����Ըû�������������ѧ������Ϊ���Ӽ������ۼ���
�ʴ�Ϊ�����Ӽ������ۼ���
(3)���������������������ƺ�ˮ��Ӧ�����ӷ���ʽΪ2Na2O2+2H2O=4Na++4OH�C+O2����
�ʴ�Ϊ��2Na2O2+2H2O=4Na++4OH�C+O2����
(4)CΪ����EΪ�ƣ��γɵļ����Ӻ�������Ų���ͬ���˵��Խ�����Ӱ뾶ԽС�������������ӵİ뾶�ɴ�С��˳����N3�C>Na+���÷�Ӧ�Ļ�ѧ����ʽΪ��Na3N+3H2O=3NaOH+NH3����
�ʴ�Ϊ��N3�C>Na+��Na3N+3H2O=3NaOH+NH3����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������з�Ӧ�ķ���ʽ����2Fe3����2I-=2Fe2����I2����Br2��2Fe2��=2Br����2Fe3�������жϸ����ʵ���������ǿ������˳����
A.Fe3����Br2��I2B.Fe3����I2��Br2
C.Br2��Fe3����I2D.Br2��I2��Fe3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�����͵�������������ʱ�������з�Ӧ��4Li+2SOCl2��4LiCl+S+SO2�������й��ж���ȷ���ǣ���ʾ��SOCl2��S��![]() �ۣ�
�ۣ�
A. ��ԭ��ֻ��Li
B. SOCl2�������������ǻ�ԭ��
C. ��ԭ�������LiCl��S
D. ����1.12LSO2ʱ����Ӧת�Ƶ���Ϊ0.2mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������(FeC6H6O7)��һ�������յĸ�Ч���Ƽ��������̷�(FeSO4��7H2O)ͨ�����з�Ӧ�Ʊ���FeSO4+Na2CO3===FeCO3��+Na2SO4 FeCO3 +C6H8O7=FeC6H6O7+CO2��+H2O
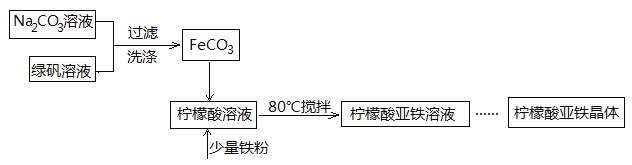
��1�����ɵ�FeCO3�����辭���ϴ�ӣ�����ϴ���Ƿ���ȫ�ķ�����____________________��
��2�����Ƶõ�FeCO3���뵽������������Һ�У��ټ����������ۣ�80���½��跴Ӧ��
�����۵�������_________________��
�ڷ�Ӧ������������ˣ���ȥ�������۵ķ�����_____________��
��3�������Һ��Ũ��������������ˮ�Ҵ������á����ˡ�ϴ�ӡ��������������������塣��������м�����ˮ�Ҵ���Ŀ����________________��
��4��ij�о���ѧϰС����������������(��Ҫ�ɷ�ΪFe2O3��SiO2��Al2O3 ) ���������Ʊ��̷����ٺϳ�������������������ͼ���̷��ܽ�����ߣ����������������������Ʊ�FeSO4��7H2O�����ʵ�鲽��(��ѡ�õ��Լ������ۡ�ϡ�����NaOH��Һ)��
����һ���������м���������ϡ�����ַ�Ӧ������
������Һ�еμӹ�����NaOH��Һ�����ˣ����ϴ�ӹ���
��������м�������ϡ������������ȫ�ܽ�
���ټ������������ۣ���ֽ�����˵õ�FeSO4 ��Һ
��_______________________________________________���õ�FeSO4��7H2O ���塣
��5��ȡ25.00g�������������壨Ħ������Ϊ246g/mol�������100mL��Һ��ȡ20.00mL����ƿ�У���ȡ0.2000mol.L-1������KMnO4����Һװ����ʽ�ζ����У���������ԭ���ⶨ����������������������������ʲ�������KMnO4����Һ��Ӧ����4�εζ���ÿ������KMnO4��Һ��������£�
ʵ����� | 1 | 2 | 3 | 4 |
����KMnO4��Һ��� | 20.00mL | 19.98mL | 21.38mL | 20.02mL |
�ζ��յ�ʱ����Ϊ��__________________________________��������������������________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������й�SO2��Cl2������ʵ�顣
(1)ijС�������ͼ��ʾ��װ��ͼ(ͼ�мгֺͼ���װ����ȥ)���ֱ��о�SO2��Cl2�����ʡ�
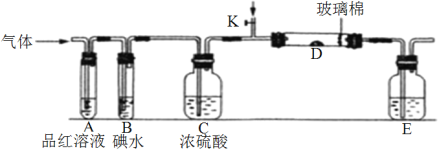
��������˷ֱ�ͨ��SO2��Cl2��װ��A�й۲쵽������_________(���ͬ����ͬ��)����װ��D��װ����V2O5(����)��ͨ��SO2ʱ����Kͨ������O2�Ļ�ѧ��Ӧ����ʽΪ__________________________________��
��SO2ͨ��B�У���Һ��ɫ��ȥ����÷�Ӧ�����ӷ���ʽΪ_______________________��
(2)ijͬѧ��������SO2ͨ��һ֧װ���Ȼ�����Һ���Թܣ�δ���������ɣ�������Թ��м�������_________(����ĸ)���ܲ�����ɫ������
A����ˮ B��ϡ���� C���������Һ D���Ȼ�����Һ
(3)�������ͨ��Cl2��д��Cl2��װ��E����Һ��Ӧ�����ӷ���ʽ____________________��
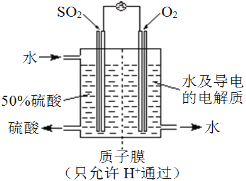
(4)ij���е�λ���õ绯ѧԭ����SO2���Ʊ����ᣬװ����ͼ������ij�ִ������缫Ϊ��IJ��ϣ����������壬ͬʱҲ��ʹ������������Һ��ֽӴ���ͨ��SO2�ĵ缫Ϊ____������缫��ӦʽΪ_____________________________����ص��ܷ�ӦʽΪ_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��H2C2O4Ϊ��Ԫ���ᡣ������0.1mol��L��1 NaHC2O4��Һ��pHԼΪ4����ָ���������ʵ���Ũ�ȹ�ϵ��������
A. c(Na+)= c(HC2O![]() )+c(C2O
)+c(C2O![]() )+c(H2C2O4)
)+c(H2C2O4)
B. c(HC2O4��)��c(H2C2O4)��c(C2O![]() )
)
C. c(Na+)+c(H+)=c(HC2O![]() )+ c(C2O
)+ c(C2O![]() )+c(OH��)
)+c(OH��)
D. c(H2C2O4)+c(H+)=c(C2O![]() )+c(OH��)
)+c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ���������ֵ��������������ȷ����( )
A. 28g���������ɵ�CO��N2��������к���ԭ����Ϊ2NA
B. ��100mL0.1mol/L��FeCl3��Һ�����ˮ�п��Ƶ�Fe(OH��3������ĿΪ0.01 NA
C. ��H2O2 + Cl2 =2HCl + O2��Ӧ�У�ÿ����32g��������ת��4NA������
D. 1L 1 mol/L ����������NA ��HCl����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ȥCl2�л��е�������HCl���壬���ѡ��
A. H2O B. ����NaCl��Һ C. Na2CO3��Һ D. NaOH��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±����и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת������
a | b | c | |
�� | Cu | CuO | Cu��OH��2 |
�� | CH3CH2OH | CH3CHO | CH2=CH2 |
�� | S | SO3 | H2SO4 |
�� | NO | NO2 | HNO3 |
�� | FeCl2 | Fe | FeCl3 |

A. �ڢ� B. �ۢ� C. �ܢ� D. �٢�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com