
����Ŀ��Cu3N�������õĵ�ѧ��ѧ���ܣ��ڵ��ӹ�ҵ�����պ�������������ͨѶ�����Լ���ѧ���̵������У������Ź㷺�ġ���������ľ����á�
(1)C��N��O����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ___________��
(2)��N3��������ͬ����������ԭ�ӷ��ӵĿռ乹����___________��
(3)Cu+�ĺ�������Ų�ʽΪ___________������������Һ�в��ȶ����ɷ����绯��Ӧ����Cu2+��Cu����CuO�ڸ����»�ֽ��Cu2O���Դӽṹ�ǶȽ�������CuOΪ�λ�����Cu2O__________________��
(4)��Cu�Ĵ������£��Ҵ��ɱ���������Ϊ��ȩ����ȩ������̼ԭ�ӵ��ӻ���ʽ��___________���Ҵ��ķе����Ը�����ȩ������Ҫԭ��Ϊ___________��
(5)[Cu(H2O)4]2+Ϊƽ�������νṹ�����е�����H2O��Cl��ȡ�������ֲ�ͬ�Ľṹ���Ի���[Cu(H2O)2(Cl)2]���м��Եķ��ӵĽṹʽ___________��
(6)Cu3N�ľ����ṹ��ͼ��ʾ��N3������λ��Ϊ___________��Cu+�İ뾶Ϊapm��N3���İ뾶Ϊbpm��Cu3N���ܶ�Ϊ___________g��cm��3(�����ӵ�������NA��ʾ)��
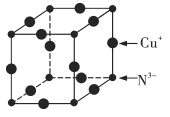
���𰸡�N��O��C V�� 1s22s22p63s23p63d10 Cu+��3d����ϵ���ȫ�������ṹ���ȶ� sp3��sp2 �Ҵ����Ӽ������� 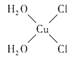 6
6 ![]()
��������
��1����һ�����ܣ�ͬ����Ԫ�آ�A>��A��(2) N3�����е�����Ϊ10������10���ӵ���ԭ�ӷ�����H2O��(3)Cu+������28�����ӣ����ݺ��ع���ԭ�ӹ����ȫ�ա�ȫ��������ʱ�ṹ�ȶ���(3)̼ԭ���γ�4������ʱ���ӻ���ʽ��sp3������˫����̼ԭ���ӻ���ʽ��sp2�����ڷ��Ӽ�����������۷е����Է�����������IJ�����������ʸߣ�(5)���Է��ӵ�����������IJ��غϣ�(6)N3������λ������N3�����ڵ�Cu+�ĸ�����Cu+�İ뾶Ϊapm��N3���İ뾶Ϊbpm�����Ծ����ı߳���![]() �����ݾ�̯����1����������Cu+��
�����ݾ�̯����1����������Cu+��![]() =3��N3����
=3��N3����![]() �������ܶȵ�����������������㡣
�������ܶȵ�����������������㡣
��1��ͬһ����Ԫ�صĵ�һ����������ԭ�����������������������ƣ�����IIA��͵�VA��Ԫ�صĵ�һ�����ܴ�������Ԫ�أ�����C��N��O����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ��N��O��C��(2)��N3��������ͬ����������ԭ�ӷ�����H2O��Oԭ�ӵ��ӻ���ʽ��sp3����2�Թµ��Ӷԣ�H2O�ռ乹����V�Σ�(3)Cu+�ĺ�������Ų�ʽ��1s22s22p63s23p63d10��CuO�ڸ����»�ֽ������ȶ���Cu2O������ΪCu+��3d����ϵ���ȫ�������ṹ���ȶ���(4)��ȩ�Ľṹ��ʽ��CH3CHO��ǰ��-CH3��C��sp3�ӻ���ȩ��-CHO�д���̼��˫�������е�̼ԭ�Ӳ�ȡ����sp2�ӻ����Ҵ����Ӽ��������������Ҵ��ķе����Ը�����ȩ��(5) [Cu(H2O)4]2+Ϊƽ�������νṹ�� [Cu(H2O)2(Cl)2]���м��Եķ��ӵĽṹʽ�� ��(6)��N3�����ڵ�Cu+��6���������ı߳���
��(6)��N3�����ڵ�Cu+��6���������ı߳���![]() �� 1����������Cu+��
�� 1����������Cu+��![]() =3��N3����
=3��N3����![]() ��������Ħ��������206g/mol��Cu3N���ܶ�Ϊ206g��NA��[(2a+2b)��10-10cm]3=
��������Ħ��������206g/mol��Cu3N���ܶ�Ϊ206g��NA��[(2a+2b)��10-10cm]3=![]() g��cm��3��
g��cm��3��


 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)���������ĸ���������ԭ�������ɴ�С��˳��������________��
��0.5 mol����
�ڱ�״����22.4 L����
��4 ��ʱ9 mLˮ
��0.2 mol������(Na3PO4)
(2)����m gij���壬������ԭ�ӷ��ӣ���Ħ������ΪM g��mol��1���������ӵ�������NA��ʾ���������Ϸ��ż���Ӧ������д���пո�
�ٸ���������ʵ���Ϊ________mol��
�ڸ���������ԭ������Ϊ________����
�۸������ڱ�״���µ����Ϊ________L��
�ܸ�������ȫ����ˮ�γ�V L��Һ(�����Ƿ�Ӧ)��������Һ�����ʵ���Ũ��Ϊ________mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з����е�����ԭ���ӻ������������ͬ����( )
A. CO2��SO2 B. CH4��NH3 C. BeCl2��BF3 D. C2H2��C2H4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����б��﷽ʽ��˵����ȷ���ǣ� ��
A. ��̬������̬�����Ĺ����Ƿ������
B. �Ȼ�淋ĵ���ʽ��
C. NH3��H2O��CO2��HCl �ķ����йµ��Ӷ������� CO2
D. �����ӵĺ�������Ų�ʽ 1s22s22p63s23p4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ� ��
A. SO2 �� CO2 �ķ������幹�;�Ϊֱ����
B. H2O �� NH3 �е�����ԭ���ӻ���ʽ��ͬ
C. CS2 Ϊ�ռ乹��Ϊ V �εļ��Է���
D. HCN��SiF4 �� SO32- ������ԭ�Ӿ�Ϊ sp3 �ӻ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ���Ȼ���������ýȾ�����л��ϳ��е��Ȼ�������ʵ���ҿ������ڵ���(�۵�232��)��Cl2��Ӧ�Ʊ�SnCl4��װ����ͼ��

��֪����SnCl2��SnCl4�й��������ʣ�

��SnCl4����ˮ������SnO2��xH2O��
�ش��������⣺
(1)����a��������_________________________________��װ��A�з�����Ӧ�����ӷ���ʽΪ____________________��
(2)���۲쵽װ��FҺ���Ϸ�___________ʱ�ſ�ʼ��ȼD���ľƾ��ƣ������ۻ����ʵ����������������������ȡ���ʱ�������ȵ�Ŀ���Ǣ�_________________________________����____________��
(3)������װ����ȱ��װ��C(��������ͬ)����D����֧�Թ��з�������Ҫ����Ӧ��ѧ����ʽΪ_________________________________��
(4)Cl2�����ķ�Ӧ������SnCl4��SnCl2��Ϊ��ֹ��Ʒ�д�������SnCl2���ɲ�ȡ�Ĵ�ʩ��____________________________________________��
(5)�ζ�������Ʒ��2��Sn(��)�ĺ������÷�����ƽ��ȡ5.000g��Ʒ����ƿ�У�������ˮ�ܽ⣬���������Һ����0.1000mol��L��1�ĵ����Һ�ζ����յ�ʱ����20.00mL�����Ʒ��Sn(��)�ĺ���Ϊ___________��(��֪Sn2++I2=2I��+Sn4+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����жԷ��ӵ����ʵĽ����У�����ȷ���ǣ� ��
A. �����������Ȼ�̼������������ˮ��������������ԭ������
B. ���ԣ�H3PO4>HClO����Ϊ H3PO4 �ķ��ǻ���ԭ������ HClO �Ķ�
C. ˮ���ȶ���1000�����ϲŻᲿ�ַֽ⣩����Ϊˮ�к��д������������
D. �����ط���ʽΪ C15H22O5���ṹ��ͼ���÷����а��� 7 ������̼ԭ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CuS��Cu2S���ڴ������Է�ˮ�е�Cr2O72-��Ӧ���¡���ӦI��CuS+Cr2O72-+H+��Cu2++SO42-+Cr3++H2O(δ��ƽ) ��Ӧ��Cu2S+Cr2O72-+H+��Cu2++SO42-+Cr3++H2O(δ��ƽ) �����й�˵����ȷ����
A. ��ӦI�͢��и���2��Ԫ�صĻ��ϼ۷����仯
B. ����1molCr2O72-ʱ��ӦI����������H+�����ʵ������
C. ��Ӧ���л�ԭ���������������ʵ���֮��Ϊ3��5
D. ������ͬʱ��Cu2S��ȥ�������Cr2O72-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ƿ��������ʪ�Թؽ��ס���������֢�����ף���ϳ�·�����£�

��֪:A����ʽΪC8H8��E�ķ���ʽΪC4H6
i.![]()
ii.
iii.
��1��A���ڷ�������������Ϊ_____________��
��2����Ӧ�ڵķ�Ӧ������____________________��
��3��B�Ľṹ��ʽ��_________________________��
��4��D�к��еĹ���������____________��
��5����Ӧ�ٵĻ�ѧ����ʽ��__________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com