
��10�֣� A��B��C��D��E��λ�ڶ����ڵ�����Ԫ�ء���֪�������ȶ��ԣ�HmD��HmC����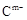 ��
��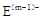 ������ͬ�ĵ��Ӳ�ṹ����A��B��ͬһ���ڣ��ڸ�������������Ԫ���У�A��ԭ�Ӱ뾶���B�����Ӱ뾶��С����A��B������֮����D��������3��������������Ϣ����Ӧ�Ļ�ѧ����ش��������⣺
������ͬ�ĵ��Ӳ�ṹ����A��B��ͬһ���ڣ��ڸ�������������Ԫ���У�A��ԭ�Ӱ뾶���B�����Ӱ뾶��С����A��B������֮����D��������3��������������Ϣ����Ӧ�Ļ�ѧ����ش��������⣺
HmDm�ĵ���ʽ___________________����1�֣�
��֤��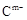 ��
��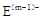 �Ļ�ԭ��ǿ�������ӷ���ʽΪ__________________________________��
�Ļ�ԭ��ǿ�������ӷ���ʽΪ__________________________________��
��3����E�ĵ���ͨ��A��D�γɵĻ������ˮ��Һ�У������ӷ���ʽΪ��__________________________��
��4�������£��������ʵ���Ũ�ȵ�HmC��Һ��A������������Ӧ��ˮ������Һ�������ϣ�д���÷�Ӧ�����ӷ���ʽ ��
�ڸ���Һ�������к��еĻ�ѧ�������� ��1�֣�
��5����A��B��C��E�����У���������ת����ϵ����_____________����Ԫ�ط��ţ���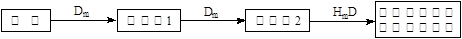
��10�֣�ÿ��2�֣�
��1�� 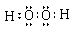 ��1�֣�
��1�֣�
��2�� Cl2 + S2��= 2Cl��+ S��
��3�� Cl2 + 2OH��= Cl��+ ClO��+ H2O
��4�� H2S+ OH��= HS��+ H2O ���Ӽ����ۼ���1�֣�
��5�� Na��S
���������������1�����ݢٿ�֪C��D��ͬ����Ԫ�أ�D�ķǽ����Ա�Cǿ������D�ǵڶ����ڣ�C�ǵ�������Ԫ�أ����ݢۢܿ�֪A�ǵ�һ����Ԫ�أ�B�ǵ�������Ԫ�أ��Ҷ��ǵ�������Ԫ�أ�����A ��Na��B��Al����D��O��C��SԪ�أ�����E��ClԪ�ء�HmDm�ĵ���ʽ����������ĵ���ʽΪ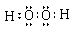 ��
��
��2��S2-�Ļ�ԭ�Ա�Cl-�Ļ�ԭ��ǿ������Ϊ��Cl2 + S2��= 2Cl��+ S�����ӷ�Ӧ������
��3��A��D�Ļ������ˮ��Һ������������Һ��E�ĵ�����������������������������Һ��Ӧ�����ӷ���ʽΪCl2 + 2OH��= Cl��+ ClO��+ H2O��
��4��A������������Ӧ��ˮ������Һ������������Һ��������ʵ�����H2S��Ӧ�������⻯�ƺ�ˮ�����ӷ���ʽΪH2S+ OH��= HS��+ H2O������Һ��������NaHS��������ѧ�������������Ӽ����ۼ���
��5��������Ԫ���п��Է�������������Ԫ����Na��S��Na��������Ӧ���������ƣ��������������������ɹ������ƣ�����������ˮ��Ӧ�����������ƣ���������ͼ��S��������Ӧ���ɶ���������������������һ�������·�Ӧ������������������������ˮ�������ᡣ
���㣺����Ԫ�ص��ƶϣ�Ԫ�ؼ��仯����Ļ�ѧ���ʣ����ӷ���ʽ������ʽ����д����ѧ�����ж�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ˮ��Һ�д��������һ��������
| A��H+��Fe3+��I����SO42�� |
| B��Al3+��Mg2+��HCO3����Cl�� |
| C��K+��Ca2+��NO3����SiO32�� |
| D��K+��Na+��OH����AlO2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���н���ʵ����ʵ�ķ�Ӧ����ʽ����ȷ����
| A��ʢ���ռ���Լ�ƿ�����ò�������SiO2+2NaOH��Na2SiO3+H2O |
| B�����ռ���Һ����������Cl2+2OH����Cl��+ClO��+H2O |
C����KSCN��Һ����Fe3+��Fe3++3SCN�� Fe(SCN)3 Fe(SCN)3 |
| D������KI������Һ���ú������4I��+O2+2H2O��2I2+4OH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��18�֣��������(K2FeO4)��һ�ּ�������������������һ������Ͷ��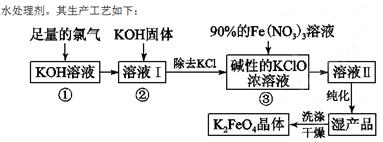
��1����Ӧ��Ӧ���¶Ƚϵ͵�����½��С������¶Ƚϸ�ʱKOH��Cl2��Ӧ���ɵ���KClO3��д�����¶Ƚϸ�ʱKOH��Cl2��Ӧ�Ļ�ѧ����ʽ ���÷�Ӧ������������ ��
��2������Һ���м���KOH�����Ŀ���� �����ţ���
| A������Һ���й�����Cl2������Ӧ�����ɸ����KClO |
| B��KOH�����ܽ�ʱ��ų��϶����������������߷�Ӧ���� |
| C��Ϊ��һ����Ӧ�ṩ���ԵĻ��� |
| D��ʹKClO3ת��ΪKClO |
 ��____H2O �� ____Fe��OH��3�����壩��____O2����____OH����
��____H2O �� ____Fe��OH��3�����壩��____O2����____OH�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣���֪��Ӧ��3NO2+H2O==2HNO3+NO���ش��������⣺
��1���÷�Ӧ�����ӷ���ʽΪ ��
��2���������뻹ԭ����������Ϊ �����������뻹ԭ��������ʵ���֮��Ϊ ��
��3���ڱ�״���£�3.36L NO2��H2O��ȫ��Ӧת�Ƶĵ�����ĿΪ ��
��4��д��HNO3��ʯ��ˮ��Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��16�֣�ʯī�ڲ�����������ҪӦ�á�ij����ʯī�к�SiO2��7.8%����Al2O3(5.1%)��Fe2O3(3.1%)��MgO(0.5%)�����ʡ���Ƶ��ᴿ���ۺ�Ӧ�ù������£�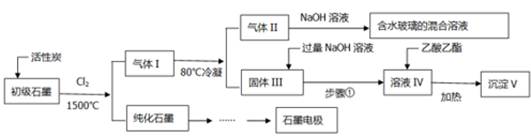
��ע��SiCl4�ķе���57.6ºC�������Ȼ���ķе������150ºC��
��1����Ӧ����ͨ��Cl2ǰ����ͨһ��ʱ���N2����ҪĿ���� ��
��2�����·�Ӧ��ʯī�е����������ʾ�ת��Ϊ��Ӧ���Ȼ������I�е��Ȼ�����ҪΪ ��������II��ij���ʵõ�ˮ�����Ļ�ѧ����ʽΪ ��
��3�������Ϊ�����衢 ��������ҺIV���������� ��
��4������ҺIV���ɳ���V���ܷ�Ӧ�����ӷ���ʽΪ ��100kg����ʯī���ɻ��V������Ϊkg ��
��5��ʯī��������Ȼˮ����ͭ���ĵ绯ѧ�����������ͼ����ʾ��ͼ��������Ӧ��ע��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ˮ�к���������[Au(CN)2]+����������CN���ж���CN-��H+�������HCNʱ���䶾�Ը�ǿ���ش��������⣺
��1��HCN�ĵ��뷽��ʽΪ______________________NaCN��Һ��pH_____7(�< > =��)
��2����������ʵĵ��뷽��ʽ���ƣ�[Au(CN)2]+Ҳ�������������룬��һ�����뷽��ʽΪ_________
��3)�������ַ�ˮ���ڼ��������£�NaClO��CN������Ϊ̼����͵����������ӷ���ʽΪ��__________
��4)�����������£�ClO��Ҳ������CN--,��ʵ�ʴ�����ˮʱȴ�������������½��е�ԭ����_______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣��������ѹ㷺Ӧ���ڸ���ṹ����Ϳ�ϡ�ֽ��Ϳ��ȣ��������ѻ�����Ϊ�Ʊ��ѵ��ʵ�ԭ�ϡ�
��.�������ѿ����������ַ����Ʊ���
����1��TiCl4ˮ������TiO2��xH2O�����ˡ�ˮϴ��ȥ���е�Cl�����ٺ�ɡ����ճ�ȥˮ�ֵõ�����TiO2���˷����Ʊ��õ��������������ѡ�
��1����TiCl4ˮ������TiO2��xH2O�Ļ�ѧ����ʽΪ ��
�ڼ���TiO2��xH2O��Cl���Ƿ����ķ����� ��
����2�����ú���Fe2O3����������Ҫ�ɷ�ΪFeTiO3������TiԪ�ػ��ϼ�Ϊ+4�ۣ���ȡ������Ҫ�������£�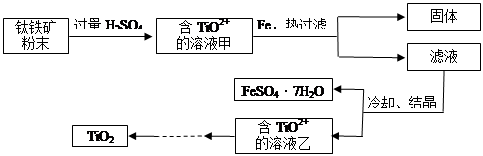
��2��Fe2O3��H2SO4��Ӧ�����ӷ���ʽ�� ��
��3������Һ�г���TiO2+֮����еĽ����������� ��
��4����Fe�������� ��
�������ѿ�������ȡ�ѵ���
��5��TiO2��ȡ����Ti���漰���IJ������£�
TiO2 TiCl4
TiCl4 Ti
Ti
��Ӧ�ڵķ���ʽ�� ���÷�Ӧ��Ҫ��Ar�����н�
�У������ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��Һ�п��ܺ���OH����CO32����AlO2����SiO32����SO42����K����Na����Fe3����Mg2����Al3�������ӣ�������Һ����μ���һ�����ʵ���Ũ�ȵ�����ʱ�����ɳ��������ʵ����������������Ĺ�ϵ��ͼ��ʾ[��֪��AlO2����HCO3����H2O=Al(OH)3����CO32��]���ش��������⣺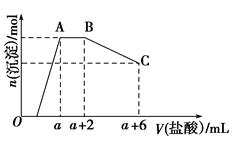
(1)ԭ�����Һ��һ�����е�������________��
(2)AB�η�Ӧ�����ӷ���ʽ��__________________________��
(3)AB�κ����ᷴӦ��������BC�κ����ᷴӦ�����ʵ����ʵ���֮��Ϊ______________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com