
����Ŀ��ʵ�����Ա���ȩΪԭ���Ʊ����屽��ȩ�ķ�Ӧ���£�
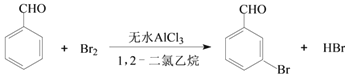
��֪��(1)���屽��ȩ�¶ȹ���ʱ�ױ�������
(2)�塢����ȩ��1,2-�������顢���屽��ȩ�ķе㼰��Է����������±���
���� | �� | ����ȩ | 1,2-�������� | ���屽��ȩ |
�е�/�� | 58.8 | 179 | 83.5 | 229 |
��Է������� | 160 | 106 | 185 |
����1����һ����ȵ���ˮAlCl3��1,2-��������ͱ���ȩ��ֻ�Ϻ�װ��������ƿ(��ͼ��ʾ)�������μӾ�Ũ��������������Һ�壬���·�Ӧһ��ʱ�䣬��ȴ��
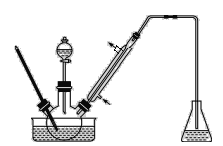
����2������Ӧ����ﻺ������һ������ϡ�����У����衢���á���Һ���л�����10% NaHCO3��Һϴ�ӡ�
����3����ϴ�ӵ��л������������ˮMgSO4���壬����һ��ʱ�����˳�MgSO4nH2O���塣
����4����ѹ�����л��㣬�ռ���Ӧ��֡�
(1)ʵ��װ���������ܵ���Ҫ������_________����ƿ��ӦΪ_____(�ѧʽ)��Һ��
(2)����1��Ӧ�����У�Ϊ���ԭ�������ʣ����˵��¶ȷ�ΧΪ(�����)_______��
A����229�� B��58.8��~179�� C����58.8��
(3)����2����10% NaHCO3��Һϴ�ӣ���Ϊ�˳�ȥ�����л����_______(�ѧʽ)��
(4)����3�м�����ˮMgSO4�����������_____________��
(5)����4�в��ü�ѹ������Ϊ�˷�ֹ__________________________________��
(6)��ʵ���м�����5.3 g����ȩ���õ�3.7 g���屽��ȩ������屽��ȩ����Ϊ______��
���𰸡��������� NaOH C Br2 ��ȥ�л����ˮ ���屽��ȩ���¶ȹ��߱����� 40%
��������
��1�������ܵ�������:��������,�����ԭ�ϵ�������,��ƿ��ʢ�ŵ���NaOH��Һ�����շе�ϵ͵���;�����Ϊ:����������NaOH ��
(2)����1��Ӧ������,Ϊ�����ԭ��������,Ӧ�����¶ȵ�����ͱ���ȩ�ķе�,�����¶�Ӧ����58.8��C;�����Ϊ:C��
(3)����2�л����е�������NaHCO3��Һ��Ӧ,�����л�����10%NaHCO3��Һϴ�ӣ���Ϊ�˳�ȥ��;�����Ϊ:Br2��
��4����ϴ�ӵ��л������������ˮMgSO4����,����-��ʱ�����˳�MgSO4![]() nH2O����,���Բ���3�м�����ˮMgSO4����,��Ϊ�˳�ȥ�л����е�ˮ;�����Ϊ:��ȥ�л����ˮ��
nH2O����,���Բ���3�м�����ˮMgSO4����,��Ϊ�˳�ȥ�л����е�ˮ;�����Ϊ:��ȥ�л����ˮ��
(5)���ü�ѹ����,���Խ��������ķе�,��˿����ڽϵ͵��¶��·�������,��ֹ���屽��ȩ���¶ȹ��߱�����;�����Ϊ:���屽��ȩ���¶ȹ��߱�������
(6)���� ����5.3g����ȩ��ȫ��Ӧ����xg���屽��ȩ������106:185=5.3:x,���x=9.25g,���Լ��屽��ȩ����3.7g/9.25g
����5.3g����ȩ��ȫ��Ӧ����xg���屽��ȩ������106:185=5.3:x,���x=9.25g,���Լ��屽��ȩ����3.7g/9.25g ![]() 100%=40%;�����Ϊ: 40%��
100%=40%;�����Ϊ: 40%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(H3PO2)��һ�־�ϸ������Ʒ����ǿ��ԭ�ԡ���֪����2P4+3Ba(OH)2+6H2O=3Ba(H2PO2)2+2PH3������H3PO2+NaOH(����)=NaH2PO2+H2O�������ƶϲ���ȷ����
A. H3PO2�ĽṹʽΪ
B. H3PO2����ǿ��ԭ�ԣ��ڿ����п��ܱ�����������
C. NaH2PO2����ʽ��
D. ÿ����1mol P4����Ӧ����ת��6mol����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪25 ��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ,�ش���������:
��ѧʽ | CH3COOH | H2CO3 | HClO |
����ƽ�ⳣ�� | Ka=1.8��10-5 |
| Ka=3.0��10-8 |
��1�����ʵ���Ũ�Ⱦ�Ϊ0.1 mol��L-1������������Һ,pH��С�������е�˳����__________(�ñ����д)��
a.CH3COONa b.Na2CO3 c.NaClO d.NaHCO3
��2��������,0.1 mol��L-1 CH3COOH��Һ��ˮϡ������,���б���ʽ�����ݱ�����__________(����ĸ)��
a. ![]() b.
b. ![]() c.
c. ![]()
d. ![]() e.
e. ![]()
��3��д�������������Һ��ͨ������������̼�����ӷ���ʽ__________��
��4��25��ʱ,CH3COOH��CH3COONa�Ļ����Һ,����û��ҺpH=6,����Һ��:c(CH3COO-)-c(Na+)=__________(��ȷ��ֵ)��
��5��25��ʱ,��a mol��L-1�Ĵ�����b mol��L-1�������Ƶ�������,��Ӧ����Һǡ��������,��a��b��ʾ����ĵ��볣��Ϊ__________
��6�������Ϊ100 mL pH=2��CH3COOH��һԪ��HX,��ˮϡ������pH����Һ����Ĺ�ϵ��ͼ��ʾ,��HX�ĵ���ƽ�ⳣ��__________(����>������=������<��)CH3COOH�ĵ���ƽ�ⳣ����
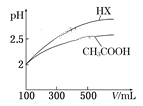
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�ִ�ֲ������ȡ����Ȼ������a-damascone��������������ˮ������ṹΪ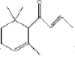 �йظû����������˵������ȷ���ǣ� ��
�йظû����������˵������ȷ���ǣ� ��
A. �û����ﺬ��2�ֹ�����
B. �û�����ɷ����Ӿ۷�Ӧ
C. �û����������ˮ����1,4-�ӳɷ�Ӧ
D. ����CCl4��Һ��Ӧ���ɵIJ��ᆳˮ�⡢ϡ�����ữ���ټ���AgNO3��Һ���ɵõ���ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������[KA1(SO4)2��12H2O]��һ�ָ��Σ�����ֽ�ȷ���Ӧ�ù㷺�����÷��������Ʊ������Ĺ�������ͼ��ʾ����������������ǣ�

A. �����������NaHSO4����NaHCO3
B. ���������пɻ��յĽ���Ԫ����Al��Fe
C. �������������������ڻ�����������Դ������
D. ����������Ӧ�Ľ�������ΪFe3+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����л�����������ȷ����
A. ![]() �Զ��ױ�
�Զ��ױ�
B. 2,3-����-3-�һ�����
C. ![]() �춡ϩ
�춡ϩ
D. (CH3)3CCH2CH(C2H5)CH3 2��2-����-4-�һ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Դ�Ŀ�������ˮ�Ĵ����������ֻ�ѧѧ�Ƶ�Ӧ�ü�ֵ��
��. ��ҵ�Ͽ�����CO2���Ʊ����ȼ�ϼ״����йػ�ѧ��Ӧ���£�
��ӦA��CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g) ��H1����49.6kJ��mol��1
CH3OH(g)��H2O(g) ��H1����49.6kJ��mol��1
��ӦB��CO2(g)��H2![]() H2O(g)��CO(g) ��H2����41kJ��mol��1
H2O(g)��CO(g) ��H2����41kJ��mol��1
(1)д����CO(g)��H2(g)�ϳ�CH3OH(g)��Ӧ���Ȼ�ѧ����ʽ�� _____________________________��
(2)д��������������߷�ӦA�м״�ƽ����ʵ�����_______________________��
(3)��Cu��ZnO��ZrO2���£�CO2��H2������壬�����1��3�������ʵ���amol���з�Ӧ�����CO2ת���ʡ�CH3OH��COѡ�������¶ȡ�ѹǿ�仯����ֱ���ͼ��ʾ��ѡ���ԣ�ת����CO2������CH3OH��CO�İٷֱȣ���

�¶ȶԷ�Ӧ��Ӱ�� ѹǿ�Է�Ӧ��Ӱ��
��������ͼ��֪��Ӱ�����ѡ���Ե����������______��
A. �¶� B. ѹǿ C. ����
��.ʵ����ģ������ӵ绯ѧ������������������ˮ��NH4+��װ����ͼ��ʾ��������狀�ȥ����ˮ���Ƴɳ�ʼ��ģ���ˮ������NaCl������Һ��������Ũ�ȣ��������ォ������ˮ�е�NH4+�����ɿ����е���Ҫ�ɷ֡�

(4)������ӦʽΪ__________________________________��
(5)��ȥNH4+�����ӷ�Ӧ����ʽΪ________________________________________��
��.�绯ѧ���ⷨ����������ˮ�������ε���Ⱦ���绯ѧ����NO3����ԭ����ͼ��ʾ��

(6)AΪ_______��������������ת��1 mol ���ӣ���Ĥ������������Һ�������仯���m�� ����m����Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���ͭ�������ַ�ˮ��Ҫ������һ�ַ�ˮ�к���CN-���ӣ���һ�ַ�ˮ�к���Cr2O72-���ӡ��ó��ⶨ��ͼ��ʾ�ķ�ˮ�������̡�

�ش��������⣺
��1������������ˮ����������Ҫʹ�õķ�����__________________
��2������ʹ�õ�NaClO��Һ�ʼ��ԣ������ӷ���ʽ����ԭ��________________��
��3�����з�Ӧ��������ų����÷�Ӧ�����ӷ���ʽΪ___________________��
��4�����з�Ӧʱ��ÿ0.4mol Cr2O72-ת��2.4mol�ĵ��ӣ��÷�Ӧ�����ӷ���ʽΪ______________��
��5��ȡ��������ˮ�����Թ��У��ȼ���NaOH��Һ���۲쵽����ɫ�������ɣ���������NaOH��Һ��ֱ�����ٲ�����ɫ����Ϊֹ���ټ���Na2S��Һ���к�ɫ�������ɣ�����ɫ�������٣�����ʹ�û�ѧ�����ϱ�Ҫ�����ֽ�����ԭ��______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���߷������������ް����������ᆳ�������䣬���������������Լ�ǿ�Ļ������ӣ����ֻ������ӿ��Էֽ��������ռ��е�һЩ�к����ʣ���ȩ����������ȣ���
��1��Ti2+�Ļ�̬�۵����Ų�ʽΪ_______________________��
��2����ȩ������Cԭ�ӹ���ӻ�����Ϊ____________��1mol��ȩ�����к�����������ĿΪ___________��
��3����ȩ������ˮ������Ϊ���Ƕ��Ǽ��Է����⣬����Ϊ_______________________��
��4����N2O��Ϊ�ȵ������һ�ַ���Ϊ________________________(�ѧʽ) ��
��5��ij���ѻ����ᄃ���ṹ��ͼ��ʾ���û�����Ļ�ѧʽΪ_____________________��
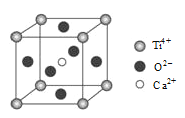
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com