
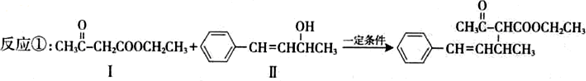
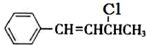 �����ɻ����������ķ�Ӧ����Ϊ
�����ɻ����������ķ�Ӧ����Ϊ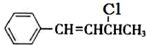 �����ɻ��������±������ˮ�ⷴӦ��
�����ɻ��������±������ˮ�ⷴӦ��| ��ȼ |
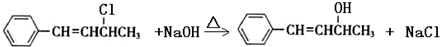 ����Ӧ����ΪNaOHˮ��Һ���ȣ�
����Ӧ����ΪNaOHˮ��Һ���ȣ�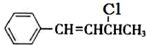 ��NaOH�Ҵ���Һ�������ɵ��л����������C6H5CH=C=CHCH3��C6H5CH=CHCH=CH2��ǰ�߲��ȶ���������IV�Ľṹ��ʽΪ
��NaOH�Ҵ���Һ�������ɵ��л����������C6H5CH=C=CHCH3��C6H5CH=CHCH=CH2��ǰ�߲��ȶ���������IV�Ľṹ��ʽΪ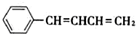 ����˴Ź������׳��������������壬�����֮��Ϊ1��2��2��1��1��1��2�����ɢ��ķ���ʽΪ
����˴Ź������׳��������������壬�����֮��Ϊ1��2��2��1��1��1��2�����ɢ��ķ���ʽΪ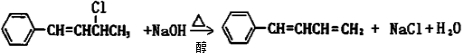 ��
�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
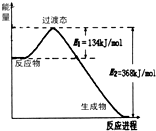 ��1����25�桢101kPa�£�1g������ȫȼ������CO2��Һ̬H2O���ų�55kJ��������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��
��1����25�桢101kPa�£�1g������ȫȼ������CO2��Һ̬H2O���ų�55kJ��������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
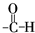
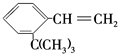 ������ˮ�����ɲ���Ľṹ��ʽΪ
������ˮ�����ɲ���Ľṹ��ʽΪ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�� ���� |
��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | ��1�� | ��2�� | ||||||
| 3 | ��3�� | ��4�� | ��5�� | ��6�� | ��7�� | ��8�� | ��9�� | |
| 4 | ��10�� | ��11�� | ��12�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ѹǿ����Ӧ���������淴Ӧ���ʼ�С��ƽ������ |
| B��ʹ�ø�Ч��������Ч�������Ӧ���� |
| C����Ӧ�ﵽƽ���N0�ķ�Ӧ���ʱ��ֺ㶨 |
| D����λʱ��������CO��CO2�����ʵ������ʱ����Ӧ�ﵽƽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ø���������ʳƷ������ |
| B���ö�������Ư������ |
| C����ţ�������������谷 |
| D����С�մ��Ƹ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com