
����Ŀ��Ŀǰ�������϶���õ�������Ȼ��Ƶķ��������������ƣ�2NaCl(����) ![]() 2Na��Cl2������֪A��B��C��D��E������ת����ϵ��
2Na��Cl2������֪A��B��C��D��E������ת����ϵ��
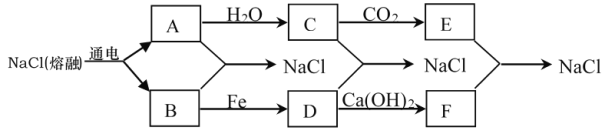
��1��д��A��B����NaCl�Ļ�ѧ����ʽ��_____________________________________��
��2��д����ѧʽ��C______________��D_____________��
��3����ҵ�����г�����B��Ca(OH)2��Ӧ���Ʊ�Ư�ۣ�Ư�۵���Ҫ�ɷ���_______________________________����д��ѧʽ��
��4������AͶ��ʢ��D����Һ�У���Һ�г���________________(�������ɫ)�������ù�����������Ӧ�Ļ�ѧ����ʽΪ____________________________________________��
���𰸡�2Na+Cl2![]() 2NaCl NaOH FeCl3 CaCl2��Ca(ClO)2 ���ɫ 2Na+2H2O=2NaOH+H2�� FeCl3+3NaOH=Fe(OH)3��+3NaCl
2NaCl NaOH FeCl3 CaCl2��Ca(ClO)2 ���ɫ 2Na+2H2O=2NaOH+H2�� FeCl3+3NaOH=Fe(OH)3��+3NaCl
��������
�����̿�֪��A��ˮ��Ӧ����C����C�������̼��Ӧ����AΪNa��CΪNaOH��EΪNa2CO3��BΪCl2��DΪFeCl3��FΪCaCl2�����Ԫ�ػ��ϼ�֪ʶ����ѧ���������
��1��AΪNa��BΪCl2������NaCl�Ļ�ѧ����ʽ��2Na+Cl2![]() 2NaCl��
2NaCl��
��2���ɷ�����֪�����ʵĻ�ѧʽ��CΪNaOH��DΪFeCl3��
��3����ҵ����ȡƯ�ۣ���Ӧ�ķ���ʽΪ��2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��Ư�۵���Ҫ�ɷ���CaCl2��Ca(ClO)2��
��4������AΪNaͶ��ʢ��DΪFeCl3����Һ�У�A��ˮ��Ӧ����NaOH��2Na+2H2O=2NaOH+H2�� ���ٷ���3NaOH+FeCl3�TFe��OH��3��+3NaCl����Һ�г��ֺ��ɫ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25�����������ε�ϡ��Һ���ֱ���![]() mol/L NaX��Һ��
mol/L NaX��Һ��![]() mol/L NaY��Һ�������жϴ�����ǣ� ��
mol/L NaY��Һ�������жϴ�����ǣ� ��
A. ��![]() �����
�����![]() ������Һ�е�
������Һ�е�![]()
B. ��![]() �����
�����![]() ������Һ�е�
������Һ�е�![]()
C. ��![]() ����
����![]() �������ԣ�
�������ԣ� ![]()
D. ��![]() �������
�������![]() ����HX��ǿ�ᣬHY������
����HX��ǿ�ᣬHY������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1�����Ե缫�������ͭ��Һ�Ļ�ѧ����ʽ_____________________________��
��2�����Ե缫��ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ_____________________________��
��ijС��ͬѧ��Ϊ�����ģ�ҵ�������ӽ���Ĥ�����ռ�ķ�������ô������������ͼװ�õ���������Һ����ȡ������������������������ء�
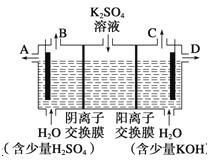
��3���õ��۵�������ӦʽΪ________________________________����ʱͨ�������ӽ���Ĥ��������________(����ڡ�����С�ڡ����ڡ�)ͨ�������ӽ���Ĥ����������
��4���Ƶõ�����������Һ�ӳ���________(��д��A������B������C����D��)������
��5�������Ƶõ�����������������������Һ���Ϊ����ȼ�ϵ�أ����������ķ�ӦʽΪ____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ������CH3CH2OH��CH3COOH��![]() ��CCl4�������ʵ��Լ��� ( )
��CCl4�������ʵ��Լ��� ( )
A��ʯ����Һ B��NaOH��Һ C������ D��H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��X��һ�־���ˮ����ζ�ĺϳ����ϣ�A��ֱ���л��E��FeCl3��Һ��������ɫ��
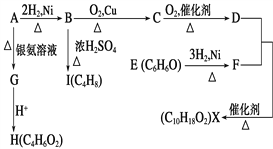
�����������Ϣ�ش�
(1)H�к��������ŵ�������_______��B��C�ķ�Ӧ����Ϊ________B��I�ķ�Ӧ����Ϊ________��
(2)ֻ��һ���Լ�����D��E��H�����Լ���________��
(3)H��J��Ϊͬ���칹�壬J������������ˮ�����������ɣ�J�Ľṹ��ʽΪ__________��
(4)D��F��Ӧ����X�Ļ�ѧ����ʽΪ_______________________��
E��F�Ļ�ѧ����ʽΪ_______________________��
B��C�Ļ�ѧ����ʽΪ_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪N��S��ClԪ�ؿ��γɶ������ʣ��ڹ�ҵ���������Ź㷺��Ӧ�á��ش��������⣺
(1)Fe3+����SCN-�γɶ��������ӣ�����һ��Ϊ[Fe(SCN)6]3-�����������е�SCN-��ʹ����ʣ��۵���ѹ����ԣ���ÿ�������������ĵ����Ӹ���Ϊ_______����
(2)Se��S��ͬ��Ԫ�أ���д����̬Seԭ�ӵ����Ų�ʽ______��N��S�Dz�ͬ��Ԫ�أ������NH3��H2S��ˮ���ܽ�ȴ��ԭ��__________________��
(3)��һ����1~9��Ԫ���еIJ���Ԫ����ɣ�����SCl2��Ϊ�ȵ�����Ĺ��ۻ�������ķ���ʽΪ__________�������ȵ�����ԭ�����Է�����SCN-�ЦҼ��ͦм��ĸ�����Ϊ__________��
(4)��֪S4O62-�ĽṹΪ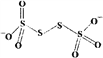 ������Sԭ�ӵ��ӻ���ʽ��______�� N��P�ɷֱ��γɶ��������ͷ��ӣ���֪NH3�ļ��Ǵ���PH3��ԭ����____________��
������Sԭ�ӵ��ӻ���ʽ��______�� N��P�ɷֱ��γɶ��������ͷ��ӣ���֪NH3�ļ��Ǵ���PH3��ԭ����____________��
(5)���Ӿ����������Ӻ������ӵİ뾶�Ȳ�ͬ���γɲ�ͬ�ľ����ṹ�����±���
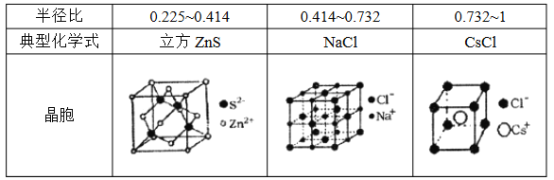
��֪ij���Ӿ���RA�����������Ӱ뾶�ֱ�Ϊ184pm��74pm��Ħ������ΪMg/mol������������λ��Ϊ_________��������ܶ�Ϊ_________g/cm3���г�����ʽ�����軯����NAΪ�����ӵ�������ֵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼʾװ����ȡ������������(�ƾ��Ƶ���ͼ�о�����ȥ)������գ�

(1)�Թ�a����Ҫ����Ũ���ᡢ��������Ҵ���2 mL����ȷ�ļ���˳������______________________��
(2)Ϊ��ֹa�е�Һ����ʵ��ʱ�������У��ڼ���ǰӦ��ȡ�Ĵ�ʩ��________��
(3)ʵ���м����Թ�a��Ŀ���ǣ���______________����______________��
(4)�Թ�b�м��б���Na2CO3��Һ����������________________��
(5)��Ӧ���������Թ�b�����á��۲쵽��������________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Һ����50 mL1mol/LAlCl3��Һ��Cl�����ʵ���Ũ����ȵ���
A. 50 mL 1 mol/LFeCl3��Һ B. 75 mL 2mol/LKCl��Һ
C. 150 mL 1 mol/LMgCl2��Һ D. 25 mL 3 mol/LCuCl2��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��XY2�����ӻ����X��Y���ӵĵ��Ӳ�ṹ������ԭ����ͬ����X��YΪ�� ��
A.Ca��Cl
B.K��S
C.Ca��F
D.Mg��F
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com