
����Ŀ����1����Ҫ��д������ʽ��
��HNO3(���뷽��ʽ) _____________��
��Fe2(SO4)3(���뷽��ʽ) ___________��
�������ƺ��Ȼ�����Һ��Ӧ(���ӷ���ʽ) ______________��
�ܶ�����̼ͨ����������������Һ(���ӷ���ʽ)_________________��
��2������Ϊ��ѧ��ѧ�г����ļ������ʣ��ٶ�����̼��������KCl����NaHSO4���塡��ͭ����ϡ���ᡡ����ʯ��ˮ���������ڵ���ʵ���_________�����ڷǵ���ʵ���________(�����)��
���𰸡�![]()
![]()
![]()
![]() �ڢ� ��
�ڢ� ��
��������
����ʵĶ�������ˮ��Һ������״̬���ܵ���Ļ�����ҵ����ԭ������Ϊ�������ĵ��룬����ʺͷǵ���ʶ������ǻ�������ʺͻ����Ȳ��ǵ����Ҳ���Ƿǵ���ʣ��ݴ˷�����
(1)��HNO3�ĵ��뷽��ʽΪ![]() ��
��
�ʴ�Ϊ��![]() ��
��
��Fe2(SO4)3�ĵ��뷽��ʽΪ![]() ��
��
�ʴ�Ϊ��![]() ��
��
�������ƺ��Ȼ�����Һ��Ӧ�����ӷ���ʽΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��
�ܶ�����̼ͨ����������������Һ�����ӷ���ʽΪ![]() ��
��
�ʴ�Ϊ��![]() ��
��
(2)�ݷ�����֪�����Ƿǵ���ʣ��ڢ��ǵ���ʣ��ܢݢȲ��ǵ����Ҳ���Ƿǵ���ʣ�
�ʴ�Ϊ���ڢۣ��٣�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͪ������ٴ�����������ʹ��ҩ��ϳ�ͪ��ҵ�һ��·�����£�

(1)F�к��������ŵ�����Ϊ____________________(д����)��
(2) ��(CH3)2SO4��ӦҲ����ֱ�ӵõ�ͪ��ң��÷�Ӧ������Ϊ____________��
��(CH3)2SO4��ӦҲ����ֱ�ӵõ�ͪ��ң��÷�Ӧ������Ϊ____________��
(3)B�ķ���ʽΪC10H11NO4��д��B�Ľṹ��ʽ��____________________��
(4)C��һ��ͬ���칹��ͬʱ��������������д����ͬ���칹��Ľṹ��ʽ��______________________��
������FeCl3��Һ������ɫ��Ӧ���ҷ�������һ������̼ԭ�ӣ�
�ڼ�������ˮ����ữ������֮һ�����������ᣬ��һˮ����������ֻ��3�ֲ�ͬ��ѧ�������⡣
(5)��֪��NO2![]() NH2��
NH2��
��д���� ��ClCH2COOC2H5Ϊԭ���Ʊ�H3COCH3CHO�ĺϳ�·������ͼ___________(���Լ����ã��ϳ�·������ͼʾ�����������)��
��ClCH2COOC2H5Ϊԭ���Ʊ�H3COCH3CHO�ĺϳ�·������ͼ___________(���Լ����ã��ϳ�·������ͼʾ�����������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��������Ͽ�ѧ�ҷ������������ᾧˮ�ľ�����5K�³��ֳ����ԡ��þ���Ļ�ѧʽΪNa0.35CoO2��1.3H2O(�þ����Ħ������Ϊ122g��mol-1)������NA��ʾ�����ӵ���������12.2g�þ����к���ԭ����Ϊ___����ԭ�ӵ����ʵ���Ϊ___mol��
��2��FeCl3��Һ����������ˮ����100mL2mol��L-1��FeCl3��Һ��ˮʱ�����ɾ��о�ˮ���õ�����___(����ڡ������ڡ���С�ڡ�)0.2NA��
��3���ڱ�״���£�VLij����(Ħ������ΪMg/mol)�ܽ���1Lˮ(ˮ���ܶȽ���Ϊ1g/cm3)�У�����������ȫ�ܽ��Ҳ���ˮ������Ӧ��������Һ���ܶ�Ϊ��g/cm3����������Һ�����ʵ���Ũ��c=___mol/L(��������ĸ��ʾ���ұ��뻯��)��
��4����ҵ�����������ƺ�ϡ����Ϊԭ���Ʊ�ClO2��Ӧ��NaClO2��HCl��ClO2����NaCl+H2O��д����ƽ�Ļ�ѧ����ʽ____��
��5����100mL��FeBr2��Һ��ͨ���״����Cl23.36L(��֪��ԭ�ԣ�Fe2����Br��)����Ӧ�����Һ��Cl����Br�������ʵ���Ũ����ȣ���ԭFeBr2��Һ�����ʵ���Ũ��Ϊ____mol/L����Ӧ�����ӷ���ʽΪ____��
��6������ʢ��10mL1mol��L��1NH4Al(SO4)2��Һ���ձ��м�20mL1.2mol��L��1Ba(OH)2��Һ����ַ�Ӧ����Һ�в������������ʵ���Ϊ____mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����ѧ��Ӧ�е������仯�����ɻ�ѧ��Ӧ�оɻ�ѧ������ʱ���յ��������»�ѧ���γ�ʱ�ų���������ͬ���¡���ͼΪN2(g)��O2(g)����NO(g)�����е������仯��

N2(g)��O2(g)����NO(g)�����е������仯
������ͨ���Ѳ�1molij��ѧ�������յ��������ɸû�ѧ���ļ��ܡ����ܵĴ�С���Ժ�����ѧ����ǿ������N��N�ļ���Ϊ________kJ/mol��
������ͼд��N2(g)��O2(g)����NO(g)���Ȼ�ѧ����ʽ��___________________________
��2������֪��C(ʯī��s)��O2(g)===CO2(g) ��H1����393.5kJ/mol
2H2(g)��O2(g)===2H2O(l) ��H2����571.6kJ/mol
2C2H2(g)��5O2(g)===4CO2(g)��2H2O(l) ��H3����2599.2kJ/mol��
����C(ʯī��s)��H2(g)��Ӧ����1mol C2H2(g)ʱ��H��________kJ/mol��
�ڻ���ƽ�����ʢ��ǿ��ԭ��Һ̬����N2H4����ǿ������Һ̬˫��ˮ������0.4molҺ̬�º�0.8mol H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7kJ������(�൱��25����101 kPa�²�õ�����)����Ӧ���Ȼ�ѧ����ʽΪ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼�������(![]() )������Ϊ��ɫ�峺Һ�塣��Ҫ����������ά�ء���֬��һЩҩ����ܼ������л��ϳɵ��м��塣��������װ���Ʊ�̼���������
)������Ϊ��ɫ�峺Һ�塣��Ҫ����������ά�ء���֬��һЩҩ����ܼ������л��ϳɵ��м��塣��������װ���Ʊ�̼���������
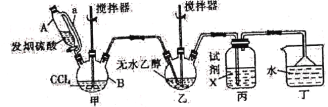
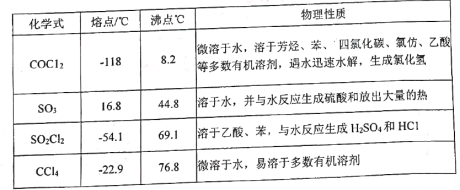
�ش��������⣺
(1)��װ����Ҫ�����Ʊ�����(COCl2)���Ƚ�����B�е����Ȼ�̼������55��60�����ٻ����μӷ������ᡣ
������B��������____________��������A�IJർ��a��������______________________��
(2)�Լ�X��____________����������_____________________________________________��
(3)��װ�ó�������COCl2�⣬�������յ�������____________(�ѧʽ)������B�����Ȼ�̼�뷢������(��SO3��ʾ)��Ӧֻ�����������ʵ���Ϊ1��1�IJ���Ҿ�����ˮ��Ӧ��д���÷�Ӧ��ѧ����ʽ��________________________________________________��
(4)��ˮ�Ҵ��������Ӧ�����ȼ����������ټ������Ҵ���Ӧ����̼���������
��д����ˮ�Ҵ��������Ӧ�����ȼ��������Ļ�ѧ����ʽ��__________________________��
������ʼͶ��92.0g��ˮ�Ҵ������յõ�̼�������94.4g����̼��������IJ�����______(��λ��Ч����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������ֵ������˵������ȷ����
A. 32 g S8�����ӽṹ��![]() ���еĹ��ۼ���ĿΪNA
���еĹ��ۼ���ĿΪNA
B. 2 g��H218O��2H2O��ɵ������к��е�������ΪNA
C. 8 g CuO������H2��ַ�Ӧ����Cu���÷�Ӧת�Ƶĵ�����Ϊ0.2NA
D. ��״���£�11.2 L Cl2����ˮ����Һ��Cl����ClO����HClO������֮��ΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ȤС���ö�п��Ƥ�����������Ʊ���ˮ������п��ZnSO4��7H2O��
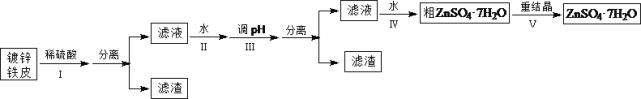
�����Ϣ���£��ٽ��������γ�����������������pH��Χ��
�������� | pH | |
��ʼ���� | ��ȫ���� | |
Fe3+ | 1.5 | 2.8 |
Fe2+ | 5.5 | 8.3 |
Zn2+ | 5.4 | 8.2 |
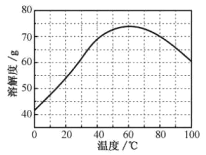
��ZnSO4���ܽ�ȣ�������100gˮ���ܽ�����������¶ȱ仯���ߡ�
��ش�
(1)�ٶ�п��Ƥ�ϵ����ۿ���Na2CO3��Һȥ����������_______________________________���ڲ���������ж϶�п����ȫ��Ӧ��ʵ��������_______________________________��
(2)�������������H2O2��������_______________________________��
(3)������ʵ�pH��Χ��_______________________________��
(4)���������Ҫ�õ���������������a.��������Һ���־�Ĥ��b.��60�������ܼ���c.��ȴ�����£�d.��100�������ܼ���e.���ˡ������������������ȷ˳��___________________���������ظ�ʹ�ã���
(5)�������ijͬѧ���ò�ͬ���·�ʽ������ȴ�ᾧ�����ZnSO4��7H2O���������С�ֲ���ͼ1��ʾ�����ݸ�ʵ������Ϊ�˵õ�������С��Ծ�һ�Ľϴ�������ѡ��_________��ʽ������ȴ�ᾧ��
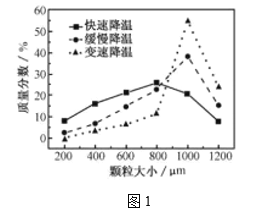
A.���ٽ��� B. �������� C.���ٽ���
(6)ZnSO4��7H2O��Ʒ�Ĵ��ȿ�����λ�ζ����ⶨ��
�� ���й��ڵζ���������ȷ����________________��
A.ͼ2�У�Ӧ����ʿ��Ϳ��������a�˺��������ڵ�c��

B.�ζ�ǰ����ƿ�͵ζ��ܾ����ñ���Һ��ϴ
C.������Һװ��ζ���ʱ��Ӧ�����ձ���©���Ȳ�������ת��
D.�ζ�ʱ��ͨ�������ֿ��������μ���Һ������ҡ����ƿ��ʹ��Һ��ͬһ������ת
E.�ζ�ǰ�ζ��ܼ����������ݣ��ζ�������������ݣ����õ������ʵ�����ĵ�С
��ͼ3����ʾ�ζ��յ�ʱ�Ķ�����_____________mL��
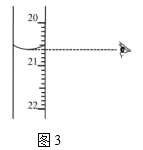
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CuCl��Ӧ�ù㷺���л��ϳɴ������ɴӻ�ͭ����Ҫ�ɷ�ΪCuFeS2������ȡ��

��֪��CuCl��һ�ְ�ɫ��ĩ������ˮ���������Ҵ����ڿ������ױ���������ˮ��Һ�д���ƽ�⣺CuCl(s)+2Cl��(aq)[CuCl3]2��(aq)����ɫ��Һ��������������ȷ����
A.����ȡ�������е���Ҫ��ӦΪCuFeS2+4CuCl2=4CuCl+FeCl2+2S
B.��ȡ���õ���FeCl2��Һ����������ʴ���ھ�Ե���ϵ�ͭ������ӡˢ��·��
C.��ˮ������CuCl(s) +2Cl��(aq)[CuCl3]2��(aq)ƽ�������ƶ�������CuCl��Cl��Ũ������
D.Ϊ��߲��ʺʹ��ȣ��ɲ����Ҵ�ϴ�ӡ���ո���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��H2NCOONH4(59��ʱ�������ֽ�)�����������м��弰ҽҩ�ȡ����ɸ����NH3��CO2�ڵ�����(���ˮԡ)��ȡ��װ����ͼ��ʾ��
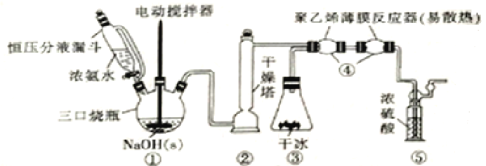
�ش��������⣺
(1)װ�â���������ѹ��Һ©����������ͨ�ķ�Һ©�������ŵ���_____________������Ũ��ˮ�������ܲ�������NH3��ԭ����____________________��װ�âڸ�������ʢ�ŵĺ��ʸ������_____________(������)��
(2)װ�â���ʢ�ɱ�װ�ô���ʵ�����Ʊ�CO2�ij����Ʊ�������ת�ã����ŵ��װ�ü���ҿɲ������������CO2�⣬�����е��ŵ���____________________________________��
(3)װ�â���������ϩ��Ĥ��Ӧ�������淴Ӧ�ܵ��ŵ���____________��������ϩ��Ĥ��Ӧ�����з�����Ӧ�Ļ�ѧ����ʽΪ_____________________________________________��
(4)�ӻ��������Ƕȷ�����װ�âݵ�������_____________��������ͨ���۲�����ȷ��NH3��CO2�ı����Ƿ���ʣ��жϵ����ݼ�������ʱ�ĵ��ڷ���Ϊ________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com