
����Ŀ����A����A����A����A �ĸ�����Ԫ�ص��⻯��ķе�仯��������ͼ��ʾ������ͼ�еĵݱ���ɣ����н�������ȷ���ǣ� ��

A. CH4 ���Ӽ���������������е�ϵ�
B. ͼ�еĺ��������ֵ��ʾ��������ԭ�ӵ���������
C. ��A ��Ԫ���⻯����ȶ�������Է��������ĵ�������ǿ
D. H2O��HF��NH3 �������ʵķе��ͬ����������⻯�ﶼ�ߣ����� Ϊ������������ʵķ��Ӽ�����γ����
 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˿Ͷ��ʢ��ϡ������Թ��У����ҷ�Ӧ�����ִ����Թ���ڸо����֡�����˵���������
A.�÷�ӦΪ���ȷ�ӦB.�÷�Ӧ�з�Ӧ��������������������������
C.�÷�Ӧ�����л�ѧ��ת��Ϊ����D.�÷�ӦΪ���ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͭ���仯�����ڿ�ѧ�о���ҵ�����о���������;����ش��������⣺
��1��������̬Cuԭ�ӵļ۵����Ų�ͼ_______________��
��2����֪������Cu2O��CuO�ȶ����Ӻ�������Ų��ǶȽ�������Cu2O���ȶ���ԭ��____________________________________��
��3�������[Cu(NH3)2]OOCCH3��̼ԭ�ӵ��ӻ�������___________���������ṩ�¶Ե��ӵ�ԭ����________��C��N��O��Ԫ�صĵ�һ�������ɴ�С��˳����_______________����Ԫ�ط��ű�ʾ����
��4��ͭ������ͭԭ�ӵĶѻ���ʽ��ͼ1��ʾ������ͭԭ�ӵĶѻ���ʽΪ____________��

��5��Mԭ�ӵļ۵����Ų�ʽΪ3s23p5��ͭ��M�γɻ�����ľ�����ͼ2��ʾ���ڵ����ͭԭ�ӣ���
�ٸþ���Ļ�ѧʽΪ_____________��
����֪ͭ��M�ĵ縺�Էֱ�Ϊ1.9��3.0����ͭ��M�γɵĻ���������________���������ӡ��������ۡ�����
����֪�þ�����ܶ�Ϊ��g��cm-3������٤������ΪNA����֪�þ�����Cuԭ�Ӻ�Mԭ��֮�����̾���Ϊ��Խ��ߵ�1/4����þ�����Cuԭ�Ӻ�Mԭ��֮�����̾���Ϊ___________pm��д��������ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�ѧ��Ӧһ���Ƿ��ȷ�Ӧ���ǣ� ��
A.Ba��OH��2��8H2O������NH4Cl������ʱ
B.��������ˮ��Ӧ
C.Ũ����ϡ��
D.����ʯ��ʯ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����мס��ҡ�������ͬѧ�ֱ������������������Ʊ�ʵ�顣
��ͬѧ����1 mol��L��1���Ȼ�����Һ�м���������������Һ��
��ͬѧ��ֱ�Ӽ��ȱ���FeCl3��Һ��
��ͬѧ����25 mL��ˮ����μ���5��6���Ȼ���������Һ�������������Һ�ʺ��ɫ��ֹͣ���ȡ�
�Իش��������⣺
(1)���в�����ȷ��ͬѧ��____________������ͬѧʵ���в�ֹͣ���ȣ��ῴ��___________��
(2)�������������Ʊ��Ļ�ѧ����ʽΪ_______________________________��
(3)֤�����������������������õĽ���������_______���ᴿ���������������峣�õķ�����________��
(4)�������������������ʵ�飺
�ٽ���װ��U�ι��ڣ���ʯī���缫��ͨ��һ��ʱ��������Դ���������ĵ缫����������ɫ������������������������__________(���������)��ɣ�
���������м��뱥����������Һ��������������________________________��
������������μ���ϡ���ᣬ������������__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС�����������ڶ�SO2�����ʽ����о�����ش��������⣺
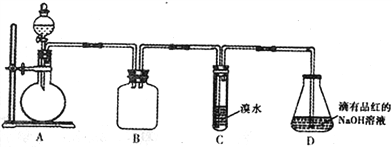
��1��װ��B��������_______________________��
��2��װ��C��Ŀ���Ǽ���SO2��_____�ԡ�װ��D��NaOHȫ��ת��ΪNaHSO3�ı�־��_______________��
��3������Ƽ�ʵ��֤������������NaHSO3��Һ��HSO3-�ĵ���ƽ�ⳣ��Ka��ˮ��ƽ�ⳣ��Kb����Դ�С��ϵ_______________________________��
��4����װ��D����NaHSO3��Һ�м���NaClO��Һʱ����Ӧ���������ֿ��ܵ������
��.HSO3-��ClO-ǡ�÷�Ӧ ��. NaClO���� ��. NaClO����
��ͬѧ�ֱ�ȡ���������Һ���Թ�����ͨ������ʵ��ȷ���÷�Ӧ������һ�������������±�������֪������H2SO3>H2CO3>HClO��
ʵ����� | ʵ����� | ���� | ���� |
�� | ���뼸С��CaCO3���� | �����ݲ��� | ���� |
�� | �μ���������KI��Һ���� | __________ | �� |
�� | �μ�������ˮ���� | _________ | �� |
�� | �μ���������KMnO4��Һ���� | ��Һ����ɫ | ______ |
��5���ⶨij���Ѿ��п��������IJ�������������SO2�������ķ���������

(��֪���ζ�ʱ��Ӧ�Ļ�ѧ����ʽΪSO2+I2 +2H2O==H2SO4+2HI)
������������ʵ�������ı�I2��Һ25.00mL���ô�ʵ������Ʒ�п��������IJ�������������SO2���㣩Ϊ_________g��L-1��
��������ʵ������������в���HI ���������������ý��________���ƫ�ߡ���ƫ�͡����䡱����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ͷ��ͨ����������ء��������̡�������ʡ�ijͬѧ��������ȼ�պ���������Ƿ���CO2����Ӧ�������з����еġ�����
A.ͨ������ʯ��ˮ��B.��ͨ������NaHCO3��Һ�У���ͨ�����ʯ��ˮ��
C.ͨ��Ʒ����Һ��D.��ͨ��������ˮ�У���ͨ�����ʯ��ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��[��ѧ����ѡ��3�����ʽṹ������]
�ѡ�������������ͭ�Ƚ������仯�����ڹ�ҵ������Ҫ��;��
(1)�����Ͻ�����ϵ����Ͻ�Ĵ������úϽ���з����¶ȵ͡��۸���е��ŵ㡣
��Cu�Ļ�̬ԭ�Ӽ۵����Ų�ʽΪ_____________��
��Ni�Ļ�̬ԭ�ӹ�___________���ֲ�ͬ�ܼ��ĵ��ӡ�
(2)�Ʊ�CrO2Cl2�ķ�ӦΪK2Cr2O7+3CCl4=2KCl+2CrO2Cl2+3COCl2��
��������ѧ����ʽ�зǽ���Ԫ�ص縺���ɴ�С��˳���ǣ�________________��(��Ԫ�ط��ű�ʾ)
��COCl2�����и�ԭ�Ӿ�����8�����ȶ��ṹ��COCl2�����ЦҼ��ͦм��ĸ�����Ϊ_______________������ԭ�ӵ��ӻ���ʽΪ_____________��
��NiO��FeO�ľ���ṹ�����Ȼ��Ƶľ���ṹ��ͬ������Ni2+��Fe2+�����Ӱ뾶�ֱ�Ϊ6.9��10-2nm��7.8��10-2nm�����۵�NiO__________FeO(�>������<����=��),ԭ����_____________��
(4)Ni��La�ĺϽ���Ŀǰʹ�ù㷺�Ĵ�����ϡ��úϽ�ľ����ṹ����ͼ��ʾ��

�ٸþ���Ļ�ѧʽΪ_____________��
����֪�þ����Ħ������ΪMg/mol,�ܶ�Ϊdg/cm3,��NAΪ�����ӵ�������ֵ����þ��������
��_____________ cm3(��M��d��NA�Ĵ���ʽ��ʾ)
�۸þ�����ڲ����п�϶����ÿ�������Ŀ����д���6����ԭ�ӱȽ��ȶ�����֪����״�����������ܶ�Ϊ��g/cm3��
��������=![]()
����������ǰ��������仯����ô�����ϵĴ�������Ϊ_______________��(��M��d���ѵĴ���ʽ��ʾ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����(Mo)��һ������ϡ�н������ҹ����ⴢ��������ڶ����⼰��Ͻ���ұ��ũҵ�������������������ȷ������Ź㷺��Ӧ�á�
��1��Mo�ɱ�������������������MoOF4��MoO2F2���ʵ�����Ϊ1:1��������з���ʽ:
____Mo+____HF+_____HNO3=_______MoO2F2+_______MoOF4+NO2��+_____________��___________
��2����֪:
��2Mo(s)+3O2(g)=2MoO3(s) ��H1
��MoS2(s)+2O2(g)==Mo(s)+2SO2(g) ��H2
��2MoS2(s)+7O2(g)==2MoO3(s)+4SO2(g) ��H3
����H3=_______(�ú���H1����H2�Ĵ���ʽ��ʾ)���ڷ�Ӧ��������0.2molMoS2�μӷ�Ӧ����ת�Ƶ���_____mol��
��3���ܱ���������Na2CO3(s)���������ͬʱ��һ������������ԭ���(MoS2)ԭ����:MoS2(s)+4H2(g)+2Na2CO3(s)==Mo(s)+2CO(g)+4H2O(g)+2Na2S(s) ��H
ʵ����ƽ��ʱ���йر仯������ͼ��ʾ
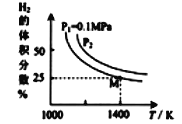
����ͼ��֪���÷�Ӧ����H____0(����>������<��)��
�����������Ӧ�����������ܱ������д�ƽ�⣬����˵���������_____(ѡ����)��
A.V��(H2)=V��(H2O)
B�ټ���MoS2����H2ת��������
C.������������ܶȲ���ʱ��һ����ƽ��״̬
D.������ѹǿ����ʱ��һ����ƽ��״̬
����ͼ��֪M��ʱ������ƽ��ת����Ϊ_____(����������0.1%)��
��ƽ�ⳣ������ƽ���ѹ����ƽ��Ũ�ȼ��㣬�����ѹ=������ѹ�����ʵ�����������ͼ��M���ƽ�ⳣ��KP=_____(MPa)2
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com