
����Ŀ����ѧ�ҷ��ֶ�ұ�����е�⾫���ᴿ�ɽ��ߴ����Ʊ��ɱ�����ص���װ����ͼ��ʾ����Cu-Si�Ͻ�����Դ����950����������Һ���ν��е�⾫�����й�˵������ȷ����
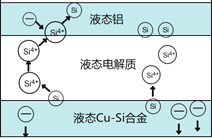
A. �ڸ�Һ��������Cu������Si��������Si4+������Cu2+����ԭ
B. ������Һ̬Cu-Si�Ͻ���������Һ̬������
C. ����Һ���ε������������ⷴӦ�������߹����Ч��
D. ����ǿ�Ȳ�ͬ����Ӱ����ᴿ����
���𰸡�A
����������ͼʾ�õ�������������ӦΪSiʧ����ת��ΪSi4+��������ӦΪSi4+�õ���ת��ΪSi������ѡ��A������ͼʾ�õ���Һ̬��Ϊ���������ӵ�Դ���������Ե��Ӵ�Һ̬�����룻Һ̬Cu-Si�Ͻ�Ϊ������������Һ̬Cu-Si�Ͻ�������ѡ��B��ȷ��ʹ������Һ���εĿ�����Ч�������ⷴӦ�������ʹ���ʹ��Ч����Һ̬���缫�ϳ�����ѡ��C��ȷ����ⷴӦ������һ���ɵ���ǿ�Ⱦ���������ѡ��D��ȷ��


 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2017������й�����֪ʶ��Ȩ��ȫ������ú���Ҵ���ҵ����ĿͶ���ɹ���ij����ú���Ҵ��Ĺ��̱�ʾ���¡�

��1��Cu(NO3)2���Ʊ�������X������Ҫ�Լ���

������A��_________��
��ʵ��������Cu(NO3)2����������Һ������������ϡHNO3�����û�ѧƽ��ԭ������HNO3������: ________��
��NaClO��Һ��������A�����ӷ���ʽ��_________��
��2������a��������3����Ҫ��Ӧ:
I.CH3COOCH3(g)+2H2(g)![]() C2H5OH(g)+CH3OH(g) ��H1
C2H5OH(g)+CH3OH(g) ��H1
II.CH3COOCH3(g)+C2H5OH(g)![]() CH3COOC2H5(g)+CH3OH(g) ��H2
CH3COOC2H5(g)+CH3OH(g) ��H2
III.CH3COOCH3(g)+H2(g)![]() CH3CHO(g)+CH3OH(g) ��H3
CH3CHO(g)+CH3OH(g) ��H3
��ͬʱ���������CH3COOCH3ת���ʡ��Ҵ�������������ѡ����(���Ҵ���ѡ����= ![]() )����ͼ��ʾ��
)����ͼ��ʾ��

����֪:��H1<0�����¶Ƚ�������ӦI��ѧƽ�ⳣ���ı仯������________��
������˵������������_______��
A.�¶ȿ�Ӱ�췴Ӧ��ѡ����
B.225��~235�棬��ӦI����ƽ��״̬
C.����H2��Ũ�ȣ��������CH3COOCH3��ת����
��Ϊ��ֹ����ӦIII����������Ӧ�¶�Ӧ���Ƶķ�Χ��____________��
����185�棬������Ӧ��CH3COOCH3��ʼ���ʵ���Ϊ5mol�������Ҵ������ʵ�����____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��������ȫ����һ�������ε�أ���ṹ��ͼ��ʾ�������йظõ�ص�˵����ȷ����

A. �ŵ�ʱO2-��a����b B. �ŵ�ʱ�����ĵ缫��ӦΪO2+4e-=2O2-
C. ���ʱ1molFe ����ΪFeOx��ת��2xmole- D. ���ʱa ���Դ�ĸ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��N ��һ����Ҫ��Ԫ�أ��䵥�ʼ�����������������ж��й㷺��Ӧ�á�
��1����̬N ԭ�ӵĺ�������Ų�ʽ��_____________������ܼ��ĵ���������ͼ��״Ϊ_____________��Nԭ�ӵĵ�һ�����ܱ�Oԭ�ӵĴ���ԭ����_________________________��
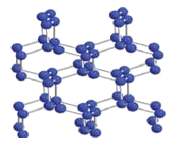
��2���ڸ�ѹ�µ����ᷢ���ۺϵõ��߾۵�������ṹ��ͼ��ʾ��������ÿ����ԭ��������������ԭ�ӽ���γɿռ���״�ṹ����ԭ�ӵ��ӻ��������Ϊ_________�����ָ߾۵�N-N ���ļ���Ϊ160kJ/mol����N2 �ļ���Ϊ942kJ/mol�������DZ�ڵ�Ӧ����______________________��
��3���Ͼ�������ѧ�Ŷӳɹ��ϳ������������ȶ����ڵ��嵪��������(N5)6(H3O)3(NH4)4Cl����X���������þ���ṹ����ֲ��ṹ��ͼ��ʾ(����N5-������ṹ��ƽ����Ԫ��)������˵����ȷ����________��
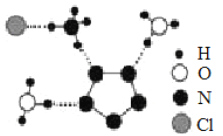
A.����N ԭ�ӵļ۵��Ӳ���й¶Ե��� B.���������Ӿ�������λ��
C.���������Ӳ��ǵȵ����� D.��������֮��ֻ�������Ӽ�
��4��NH3 ��F2 ��Ӧ����NF3 ��NH4F�������������У��е��ɸߵ��͵�˳����______��NF3�е�Ԫ����_______���������Է��ӵ���_________________��
��5�������������Ϊ��Ӳ���ϡ������ṹ��ͼ��ʾ:
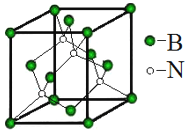
��ԭ�ӵ���λ����__________������������Ϊanm��������ܶ�Ϊ____g��cm3 (��NA ��ʾ����٤��������ֵ���г�����ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧϰС������ͼ��ʾװ�òⶨ��þ�Ͻ������������������������ԭ��������

��1��A���Լ�Ϊ____________________��
��2��ʵ��ǰ���Ƚ���þ�Ͻ���ϡ���н���Ƭ�̣���Ŀ����____________________��
��3����������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�ú�����еIJ������У�
�ټ�¼C��Һ��λ�ã�����A��B�еμ������Լ���
�۽�B��ʣ�������ˣ�ϴ�ӣ�������أ�
�ܴ�B�в� ��������������ָ������º�¼C��Һ��λ�ã�
����������˳����_________(�����)����¼C��Һ��λ��ʱ��Ӧ________________ ������ʱ����_______��
��4��B�з�����Ӧ�����ӷ���ʽΪ____________________________________ ��
��5����ʵ������þ�Ͻ������Ϊm g������������Ϊa mL(�ѻ���Ϊ��״��)�� B��ʣ����������Ϊn g�����������ԭ������Ϊ_____
��6��ʵ������У���δϴ�ӹ������õIJ����������������������________��(�ƫ����ƫС������Ӱ�족)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���鼰�仯�����ڹ�ҵ��������;�㷺��ij�о�С���ø�ѡ���Ļ������Ҫ�ɷ���Bi2S3����������SiO2�����ʣ��Ʊ�NaBiO3�����������£�
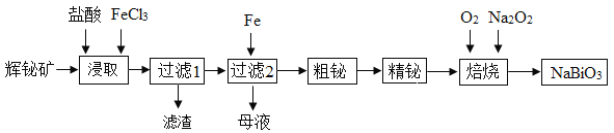
��֪��ˮ��������Bi3+��Fe3+��
�ش��������⣺
��1������ȡ��ʱ��Ϊ����߽�ȡ���ʣ��ɲ�ȡ�Ĵ�ʩ��______����дһ����������1����������Ҫ�ɷ���______���ѧʽ����
��2����ȡʱ�������Ũ�����Ŀ����______����ĸҺ����ͨ������X���ѭ�����ã�����XΪ______�������ƣ���
��3��д������ʱ���������ƵĻ�ѧ����ʽ______�������ı�״����4.48 L O2ʱ��ת�Ƶ��ӵ���Ŀ��______��
��4��25��ʱ����Ũ�Ⱦ�Ϊ0.04 mol��L��1��Cu2����Pb2����Bi3���Ļ����Һ�еμ�(NH4)2S��Һ������Һ�������1��������c(Pb2+)=10-5 mol��L-1ʱǡ����ȫ������������Һ��c(Cu2+)��c(Bi3+)��______��[��֪��Ksp(CuS)��6.0��10��36��Ksp(PbS)��3.0��10��28��Ksp(Bi2S3)��1.6��10��20]
��5����˫����(H2Dz����Ԫ���ᣩ��CC14�����ȡ���ɴӹ�ҵ��ˮ����ȡ�������ӣ�H2Dz�Ƚ�����������ϳɵ����Ե�����[��Cu(HDz)2��]������CCl4��ȡ��������ͼ��������������������Hg2+��Bi3+��Zn2+�ķ�ˮʱ��������ߣ�E%��ʾ�����������������ʽ����ȡ����İٷ��ʣ���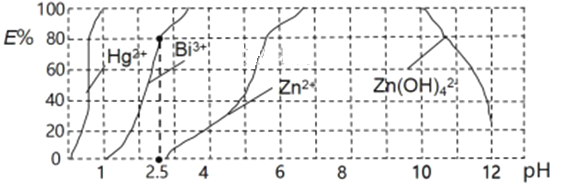
�ٵ�����pH��2.5ʱ���飨Bi���Ĵ�����ʽ��______��
������ȡ���CCl4�м���NaOH��Һ�ɽ���Ԫ���������������ʽ������������Ӧ�����ӷ���ʽΪ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������п������������������������Լ�����DZ������ϳ�·����ͼ��ʾ��
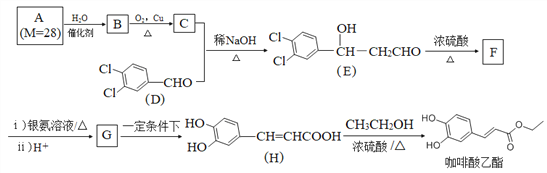
�ش��������⣺
��1��C+D��E��Ӧ����Ϊ______��F�к��������ŵ�������______��
��2��D������λ��ͬһƽ���ϵ�ԭ�������______����G�Ľṹ��ʽΪ______��
��3��H�������������Ļ�ѧ����ʽΪ______��
��4�������廯����M��H��ͬ���칹�壬1 molM������̼��������Һ��Ӧ����2 molCO2��M�Ľṹ��______�֣����к˴Ź�������Ϊ5��壬�������Ϊ1��2��2��2��1�Ľṹ��ʽΪ______��
��5������ͼ�е�C�ͼ�ȩΪԭ������ƺϳ�C5H12O4��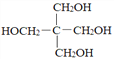 ����·�������Լ�����ѡ����________________________
����·�������Լ�����ѡ����________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�ᴿ��������(�����ڵ�����Ϊ����)����ѡ�õij����Լ�����뷽������ȷ����
ѡ�� | �ᴿ������ | �����Լ� | ���뷽�� |
A | ��Ȳ(����) | ����ͭ��Һ | ϴ�� |
B | ��(����) | Ũ��ˮ | ���� |
C | �Ҵ�(����) | ��ʯ�� | ���� |
D | ������(����������) | ˮ | �ؽᾧ |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���ҿ���KMnO4��Ũ���ᷴӦ��ȡ��������仯�ɱ���Ϊ��2KMnO4��16HCl(Ũ) ===2KCl��2MnCl2��5Cl2����8H2O��
��1���뽫������ѧ����ʽ��дΪ���ӷ���ʽ________________��
��2��Ũ�����ڷ�Ӧ����ʾ������������________________(��д���)��
��ֻ�л�ԭ���ڻ�ԭ�Ժ����� ��ֻ���������������Ժ�����
��3���˷�Ӧ������������________���ѧʽ��������0.5 mol Cl2����ת�Ƶĵ��ӵ����ʵ���Ϊ______mol��
��4����˫���ŷ���ʾ�÷�Ӧ�ĵ���ת�Ƶķ������Ŀ_______��
2KMnO4��16HCl(Ũ) ===2KCl��2MnCl2��5Cl2����8H2O
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com