
����Ŀ��������ͼ��ʾ����װ�����һ��������ⱥ��ʳ��ˮ���ⶨ���ʱ����������������ͼ��������������Ե�ʵ��װ�á�
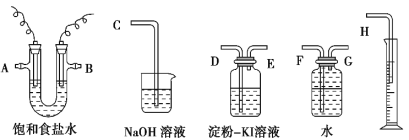
��1����ѡ���� ����ʱ�����ӿڵ�˳����(����ӿڵĴ�����ĸ)��A �� �� �� ��B �� �� �� ��_____________
��2��ʵ �� ʱ �� װ �� �� �� ʯ ī �� �� �� �� Դ �� _____�� �� �� �� �� �� �� �� �� Ӧ ʽ Ϊ_____�����缫�ӵ�Դ��_____�����������ĵ缫��ӦʽΪ_____�� �˵���ܷ�Ӧ����ʽΪ_________��
��3��ʵ���ò������������(������ɱ�״��)Ϊ 5.60 mL��������Һ�����ǡ��Ϊ50.0 mL������Һ�� OH����Ũ��Ϊ_____��
���𰸡�GFHDEC��2Cl����2e��===Cl2����2H����2e��=H2��2NaCl��2H2O![]() 2NaOH��H2����Cl2��c(OH��)��0.01 mol��L��1
2NaOH��H2����Cl2��c(OH��)��0.01 mol��L��1
��������
��1��U�ܷ�Ӧ���е������缫δ���ĸ�����������������������ѡ�á�����Ӧ������������Щ������������˳��ȡ����A��BΪ���ֵ缫����缫�����ʵ��Ҫ����A�ϵ缫Ϊ���ʵ缫��B�ϵ缫Ϊʯī�缫����Ӧ��������ѡ�õĸ������ӿ�����˳��ΪA������ƿ��G�D��F����ˮ������Ͳ��H���ܣ�����Ͳ�������ų���ˮ�����Բⶨ���������������B��ϴ��ƿ��D�D��E�����ɵ�������ϴ��ƿ���������۵⻯����Һ����֤���������ԣ����������ͨ���ձ����C���ܣ�������������������������ֹ��Ⱦ�������ʴ�Ϊ��G��F��H��D��E��C����2�����ݵ�ⱥ��ʳ��ˮ�����������ķ�Ӧʽ��2Cl����2e��===Cl2����Ϊ��ֹ�缫����ʴ��ʵ����һ��ѡ��ʯī��������������������ˮ�������H�����ӱ���ԭ��2H����2e��===H2�����Ӷ��ƻ�ˮ�ĵ���ƽ�⣬�������������γ��������ƣ��Լ��ԣ�����ͨ��ʹ�����缫�������ܷ�Ӧ����ʽΪ��2NaCl��2H2O![]() 2NaOH��H2����Cl2�����ʴ�Ϊ������2Cl����2e��===Cl2��������2H����2e��===H2����2NaCl��2H2O
2NaOH��H2����Cl2�����ʴ�Ϊ������2Cl����2e��===Cl2��������2H����2e��===H2����2NaCl��2H2O![]() 2NaOH��H2����Cl2������3��
2NaOH��H2����Cl2������3��
��֪����������5.60 mL�������ʵ���Ϊ![]() ��2��5��10��4mol���ɷ���ʽ�ɵ�
��2��5��10��4mol���ɷ���ʽ�ɵ�
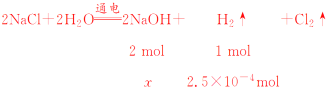
x��2��2.5��10��4mol��5��10��4mol
c(OH��)��![]() ��0.01 mol��L��1���ʴ�Ϊ��0.01 mol��L��1��
��0.01 mol��L��1���ʴ�Ϊ��0.01 mol��L��1��


 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����淴Ӧ2A+2B![]() 3C+D��ͬ�����µķ�Ӧ���ʣ���Ӧ����������( )
3C+D��ͬ�����µķ�Ӧ���ʣ���Ӧ����������( )
A. v(A)= 0.55mol (L��min) B. v(B)= 0.6mol/(L��s)
C. v(C)=0.75mol/(L��min) D. v(D)== 0.4mol/ (L��min)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ֻ��һ���Լ������,��ȥ���������е�����(������Ϊ����)д���Լ�����������ơ��������йصĻ�ѧ����ʽ�����ӷ���ʽ:
(1)Fe2O3(Al2O3)_________________;���ӷ���ʽ__________��
(2)Fe2O3[Fe(OH)3]________;��ѧ����ʽ_______________��
(3)FeSO4��Һ(CuSO4)_____________;���ӷ���ʽ_________��
(4)FeCl3��Һ(FeCl2)_____________;���ӷ���ʽ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��a��b��c��d��Ϊʯī�缫��ͨ����е�⣬����˵����ȷ����(�� ��)
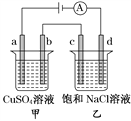
A. ��·�е�����������d��c��b��a������
B. a��c����������������ʵ������
C. SO42-��b�缫�˶���Cl-��c�缫�˶�
D. ͨ������ձ������̪��Һd������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾΪһ����AlCl3��Һ�м���NaOH��Һ����Al(OH)3��ɫ������������NaOH�����ʵ���֮��Ĺ�ϵ���ߡ�

��ش��������⣺
��1��A��ʱ�Ѳμӷ�Ӧ��AlCl3��NaOH�����ʵ���֮��Ϊ________��
��2��AB����������ʾ�ķ�Ӧ�����ӷ���ʽΪ_________________________��
��3����B�����ɵ���Һ��ͨ�������̼���ɹ۲쵽��������_______________________��
��4������0.1 mol NH4Al(SO4)2����Һ����μ���5 mol��L��1 NaOH��Һ����ʼ������Һ�г��ְ�ɫ�����������ࣻһ��ʱ����д̼�����ζ�������ݳ�������ɫ�������ٲ�������ʧ��������ͼ�л������ɳ��������ʵ��������NaOH��Һ����Ĺ�ϵʾ��ͼ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijУ��ѧ��ȤС������ͼ��ʾ���̳�ȥAlCl3�к��е�Mg2+��K+�������Ӳ������ܼ���AlCl3����ʧ����ش��������⣺
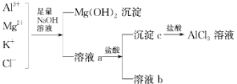
(1)д��������м�����������������Һʱ����Һ�з�����Ӧ�����ӷ���ʽ��_______________________��
(2)����������Һ�ܷ��ð�ˮ���棬Ϊʲô��___________________________��
(3)��Һa�д��ڵ�������________________������Һa�м�������ʱ��������������Ϊʲô��__________________________________��Ϊ�ˣ��Ľ�������___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵���д�����ǣ� ��
����ѧ�������Ƶ��л�����ͬϵ��
������������һ����CH2ԭ���ŵ��л�����ͬϵ��
��������̼����Ԫ�ص�����������ͬ�����DZض���ͬϵ��
����Ϊͬ���칹��������л�������������в�𣬵���ѧ���ʱض����ƣ�
A. �٢ڢ� B. �ڢ� C. �ۢ� D. �٢ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ�������йص������Һ��˵����ȷ����( )
A. ��Na2CO3��Һ��ˮϡ�ͺ�pH���Kw����
B. ����AgCl����ı�����Һ�м�����ˮ��c(Ag+)��Ksp(AgCl)������
C. pH=4.75Ũ�Ⱦ�Ϊ0.1mol/L��CH3COOH��CH3COONa�Ļ����Һ�У�c(CH3COO��)+c(OH��)<c(H+)+c(CH3COOH)
D. �ֱ���pH=2��pH=3 �� CH3COOH��Һ�к͵����ʵ�����NaOH������CH3COOH��Һ������ֱ�ΪVa��Vb����10Va=Vb
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijʵ��С����100 mL 0.50 mol��L��1 NaOH��Һ��100 mL 0.55 mol��L��1��������к��ȵIJⶨ��װ����ͼ��ʾ���ش��������⣺
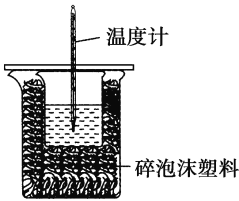
��1����ʵ�鹲��Ҫ400 mL NaOH��Һ��ʵ���������Ƹ���Һʱ������Ҫ����NaOH����______g��
��2��ͼ��װ��ȱ�ٵ�������______________________________��
��3�������Թ�����ԭ����________________________________��
��4������ĭ���ϼ���ĭ���ϰ��������___________________��
��5�����õ�Ũ�ȵĴ�����NaOH��Һ��Ӧ�����õ��к���(��H)________(����ƫ������ƫС������������)����ԭ����_______��
��6������д�±��е�ƽ���¶Ȳ
ʵ����� | ��ʼ�¶�T1/�� | ��ֹ�¶� T2/�� | ƽ���¶Ȳ� (T2��T1)/�� | |||
HCl | NaOH | ƽ��ֵ | ||||
1 | 26.2 | 26.0 | 26.1 | 30.1 | ________ | |
2 | 27.0 | 27.4 | 27.2 | 33.3 | ||
3 | 25.9 | 25.9 | 25.9 | 29.8 | ||
4 | 26.4 | 26.2 | 26.3 | 30.4 | ||
��7������ø÷�Ӧ�ų�������Ϊ2.865 kJ����д��������NaOH��Һ��Ӧ���к��ȵķ���ʽ��__________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com